Abstract
Background
The mechanism by which various classes of medication reduce COPD exacerbation risk remains unknown. We hypothesized a correlation between reduced exacerbation risk and improvement in airway patency as measured according to FEV1.
Methods
By systematic review, COPD trials were identified that reported therapeutic changes in predose FEV1 (dFEV1) and occurrence of moderate to severe exacerbations. Using meta-regression analysis, a model was generated with dFEV1 as the moderator variable and the absolute difference in exacerbation rate (RD), ratio of exacerbation rates (RRs), or hazard ratio (HR) as dependent variables.
Results
The analysis of RD and RR included 119,227 patients, and the HR analysis included 73,475 patients. For every 100-mL change in predose FEV1, the HR decreased by 21% (95% CI, 17-26; P < .001; R2 = 0.85) and the absolute exacerbation rate decreased by 0.06 per patient per year (95% CI, 0.02-0.11; P = .009; R2 = 0.05), which corresponded to an RR of 0.86 (95% CI, 0.81-0.91; P < .001; R2 = 0.20). The relationship with exacerbation risk remained statistically significant across multiple subgroup analyses.
Conclusions
A significant correlation between increased FEV1 and lower COPD exacerbation risk suggests that airway patency is an important mechanism responsible for this effect.
Key Words: COPD exacerbations, COPD mechanisms, COPD pharmacology
Abbreviations: BD, bronchodilator; dFEV1, between-group difference in change in predose (trough) FEV1 from the beginning to the end of the trial; HR, hazard ratio; non-BD, nonbronchodilator; PDE4, phosphodiesterase-4; RD, rate difference; RR, rate ratio
COPD is characterized by recurrent exacerbations, which increase in frequency with progression to worse spirometric stages of COPD.1 Exacerbations have long-term health consequences, including declines in lung function, physical status, quality of life, and survival.2, 3, 4, 5 Exacerbations also have financial consequences: hospitalizations for COPD exacerbations account for > 50% of the direct treatment costs of COPD.6 Thus, preventing exacerbations is a major objective in the treatment of COPD.7
Several classes of medications have been shown to reduce exacerbations, but the mechanism by which these different classes exert this effect is not well understood. Possibilities include symptom relief (reducing exacerbation reporting), improved airway patency (promoting clearance of secretions and reducing bacterial colonization), or a true antiinflammatory action of the treatment.
Using data from previously published clinical trials, we explored the relationship between improved airway patency, as measured by therapeutic changes in the FEV1, on COPD exacerbations. Using meta-regression analysis, we examined the hypothesis that a reduction in exacerbation risk was related to an improvement in FEV1 regardless of drug class.
Materials and Methods
As an extension of meta-analysis, meta-regression seeks to explore and quantify the effects of moderator variables on the between-trial variance in outcomes.8 For the present study, we examined the effect of change in predose FEV1 as a moderator variable on various exacerbation-related outcomes.
Identification and Selection of Trials
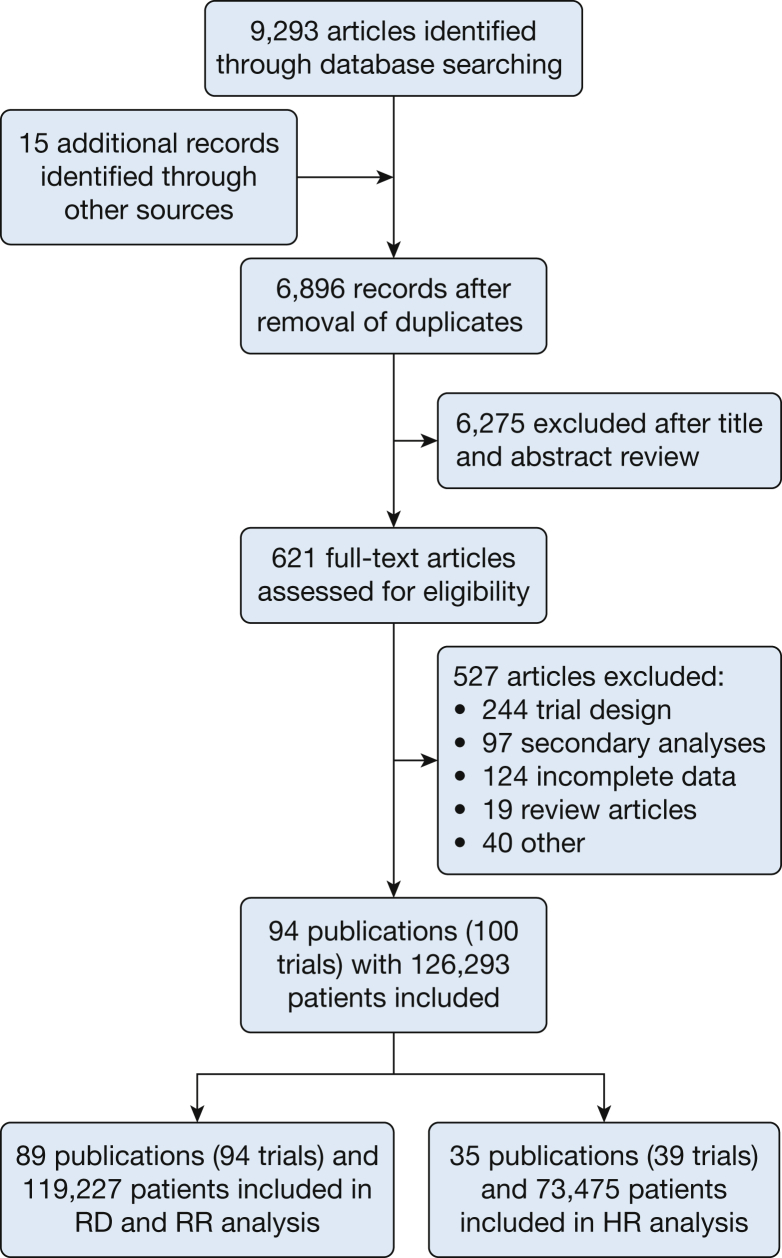
On August 5, 2015, we searched MEDLINE, clinicaltrials.gov, the Cochrane Library of Controlled Trials (CENTRAL), the Cochrane Database of Systematic Reviews, and the Cochrane Database of Abstracts of Reviews of Effects. This search yielded 6,896 results after 2,397 duplicates were removed (Fig 1). Prospective randomized trials of pharmacologic interventions at least 24 weeks in length were included that reported at least one of the following: number of exacerbations, exacerbation rates, or hazard ratio (HR); they also had to report changes in FEV1 from the beginning to the end of the study. Exacerbations and grading of their severity had to be clearly defined; the trial was otherwise excluded. Two authors (A. D. Z. and R. G. B.) assessed the citations for eligibility in the analysis. A total of 621 full-text articles were retrieved, 94 of which were included in the meta-analysis.
Figure 1.
Flow of citations in accordance with the Preferred Reporting Items for Systematic Reviews and Meta-Analyses guidelines. HR = hazard ratio; RD = rate difference; RR = rate ratio.
Data Abstraction and Outcomes
For FEV1 data, predose FEV1 data were extracted because this variable is consistently reported across studies and likely represents a longer term effect of therapy on airway patency. Predose (or trough) FEV1 is measured 23 to 24 h following drug administration.
Because the study goal was to explore the effects of therapeutic changes on exacerbations, we defined the moderator variable, dFEV1, as the between-group change from baseline in predose FEV1. Thus, for a trial with an intervention arm (a) and a control arm (b), dFEV1 = dTrial(a) – dTrial(b), where dTrial(x) represents the change from baseline for the respective arm (e-Fig 1). When possible, the dFEV1 reported by the trial was used. If necessary, dFEV1 was calculated from either the reported change in baseline for each arm (dTrial) (preferred) or the FEV1 at the beginning (dRand) and end of the trial (dEnd). If dEnd and dRand are reported, then dFEV1 = dEnd – dRand. If trials had > 1 treatment arm, only the comparisons vs the control arm (preferably placebo) were used. To examine the effects of multiple comparisons vs the same control, a subgroup analysis of the two-arm studies was also performed.
For exacerbation data, moderate to severe exacerbation rates and risk were examined. Moderate exacerbations were defined as those requiring oral corticosteroids, antibiotics, or both, and severe exacerbations were defined as those requiring hospitalization. If reported, exacerbation rates were used based on Poisson or negative binomial analysis. If only numbers of exacerbations were reported, we calculated a mean exacerbation rate (e-rate), by using the arithmetic mean number of patients observed, as follows: total exacerbations/[(patients at entry + patients at completion)/(2 × duration of study in years)].
Absolute differences in exacerbation rate (rate difference [RD]) were defined as the exacerbation rate with treatment A less the exacerbation rate with treatment B. Thus, RD = e-rate(A) – e-rate(B). Rate ratios (RR) were calculated as the rate with treatment A divided by the rate with treatment B: RR = rate(A)/rate(B). The HRs extracted were based on survival analyses of time to first moderate or severe exacerbation.
Statistical Analysis
Mixed effect meta-regression models were used to examine the impact of the moderator variable dFEV1 on the heterogeneity in three study effect estimates: RR, RD, or HR.8 A pseudo R2 statistic, which was the proportion of variance among the effect sizes that can be accounted for by the moderator, was computed for each regression model.9 Bubble scatterplots were used to represent predictions for RD, RR, and HR as a function of dFEV1 observed at the study level.10
For each clinical outcome, multiple subgroup meta-regression analyses were performed. Subgroups were examined based on trial characteristics: whether an exacerbation history was required or if a study had only two arms. We also examined subgroups of similar drug classes. Long-acting muscarinic antagonists and long-acting beta-agonists were grouped as bronchodilators (BD), and all other medications were grouped as “nonbronchodilators” (non-BD). Studentized deleted residuals were calculated for each included study to identify potential outlier studies.
For all statistical investigations, hypothesis testing was two-sided, and P values < .05 were deemed statistically significant. All statistical analyses were performed by using SAS version 9.4 (SAS Institute, Inc.) and R Version 3.2.2 (R Foundation for Statistical Computing).11
Additional details of our methods are given in e-Appendix 1.
Results
In total, 119,227 patients across 94 trials were included in the analyses of RD and RR, and 73,475 patients across 39 trials were included in the analysis of HR. Summary study characteristics and a list of the included trials can be found in e-Tables 1 and 2, respectively.
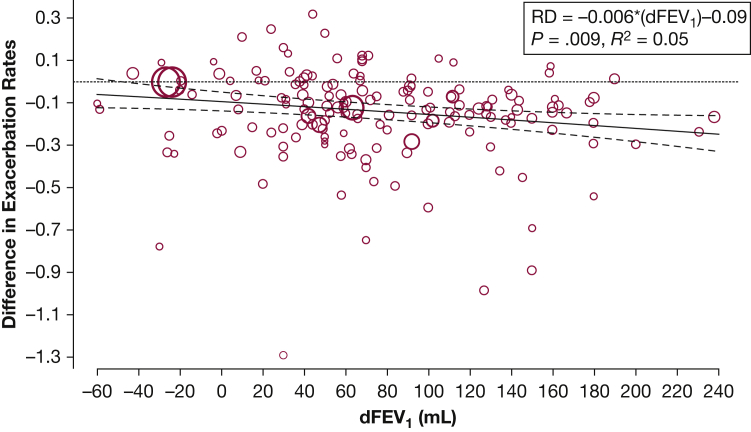
First, we examined the effect of changes in FEV1 on RD (Fig 2). For every 100-mL increase in FEV1, the number of moderate to severe exacerbations decreased by 0.06 exacerbation per patient per year (95% CI, 0.02-0.11; P = .009; R2 = 0.05). For BD-only comparisons, a 100-mL improvement correlated with 0.12 fewer exacerbation per patient per year (95% CI, 0.05-0.19; P = .001; R2 = 0.15), and for patients with at least one exacerbation in the previous year, there were also 0.12 fewer exacerbation per patient per year (95% CI, 0.01-0.24; P = .038; R2 = 0.11) (Table 1). In this part of the analysis, in which dFEV1 explained 5% to 15% of the between-trial variance of RD, we found no significant associations for inhaled corticosteroid, phosphodiesterase-4 (PDE4) inhibitors, or for all non-BD trials as a whole.
Figure 2.
Effect of dFEV1 on exacerbation RD. Each comparison is represented by a circle. The circle sizes are proportional to the contribution of individual studies toward the linear prediction, which was the inverse of the SE. The regression line is in solid black with 95% CIs shown. The unit for dFEV1 in the regression equation is 100 mL. dFEV1 = between-group difference in change in predose (trough) FEV1 from the beginning to the end of trial; RD = rate difference.
Table 1.
Effect of dFEV1 on the Absolute Difference in Exacerbation Rates
| Inclusion | No. of Trials | No. of Comparisons | No. of Subjects | Slope (b) Estimate (95% CI) |
P | R2 |
|---|---|---|---|---|---|---|
| All studies | 94 | 167 | 119,227 | –0.06 (–0.11 to –0.02) | .009 | 0.05 |
| Only two arms | 49 | 49 | 45,371 | –0.14 (–0.26 to –0.02) | .018 | 0.14 |
| BD only | 49 | 76 | 80,718 | –0.12 (–0.19 to –0.05) | .001 | 0.15 |
| ICS only | 27 | 35 | 32,354 | –0.14 (–0.29 to 0.01) | .062 | 0.13 |
| PDE4 only | 13 | 14 | 14,523 | –0.15 (–0.62 to 0.32) | .542 | 0.00 |
| Non-BD | 57 | 91 | 56,715 | –0.02 (–0.09 to 0.05) | .519 | 0.00 |
| ≥1 Exacerbation in the previous year | 27 | 47 | 31,055 | –0.12 (–0.24 to –0.01) | .038 | 0.11 |
For this analysis, the model is rate difference (RD) = a + b*dTreat. “Comparisons” represent an individual experimental vs control comparison within a trial, and “trials” represent the total number of trials. Thus, trials with > 2 arms will have > 1 comparison. The unit for dTreat is 100 mL. BD = bronchodilator; dFEV1 = between-group difference in change in predose (trough) FEV1 from the beginning to the end of the trial; ICS = inhaled corticosteroid; non-BD = nonbronchodilator; PDE4 = phosphodiesterase-4.
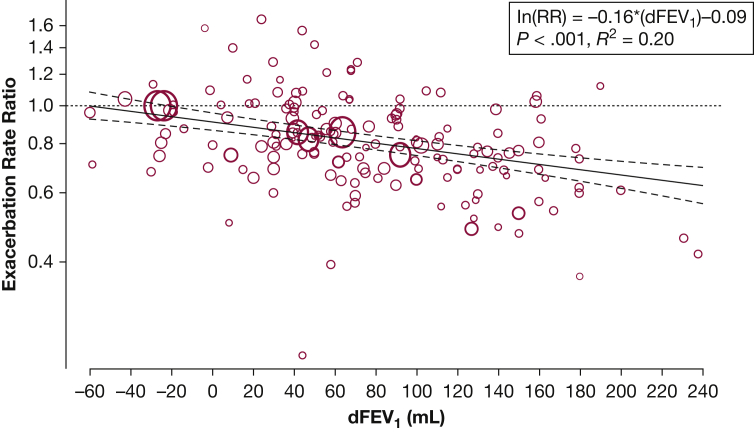
We next assessed the effect of dFEV1 on RR (Fig 3). For every 100-mL increase in FEV1, the rate of exacerbations was reduced by 14% (RR = 0.86; 95% CI, 0.81-0.91; P < .001; R2 = 0.20). For BD-only comparisons, the rate was reduced by 21% (RR = 0.79; 95% CI, 0.73-0.86; P < .001; R2 = 0.39). We also found a significant association for all non-BD trials but not for inhaled corticosteroid, PDE4 inhibitors alone, or for trials that required ≥ 1 exacerbation in the year preceding study enrollment (Table 2).
Figure 3.
Effect of dFEV1 on exacerbation RR. Each comparison is represented by a circle, and the regression line is in solid black with 95% CIs shown. The y-axis is the natural logarithm of the RR. The unit for dFEV1 in the regression equation is 100 mL. RR = rate ratio. See Figure 2 legend for expansion of other abbreviation.
Table 2.
Effect of dFEV1 on the Absolute Difference in Exacerbation Rates
| Inclusion | No. of Trials | No. of Comparisons | No. of Subjects | Slope (b) Estimate (95% CI) |
RR = Exp(b) Estimate (95% CI) |
P | R2 |
|---|---|---|---|---|---|---|---|
| All studies | 94 | 167 | 119,227 | –0.16 (–0.21 to –0.1) | 0.86 (0.81 to 0.91) | < .001 | 0.20 |
| Only two arms | 49 | 49 | 45,371 | –0.19 (–0.31 to –0.07) | 0.82 (0.73 to 0.93) | .002 | 0.24 |
| BD only | 49 | 76 | 80,718 | –0.23 (–0.31 to –0.16) | 0.79 (0.73 to 0.86) | < .001 | 0.39 |
| ICS only | 27 | 35 | 32,354 | –0.12 (–0.25 to 0.01) | 0.89 (0.78 to 1.01) | .078 | 0.18 |
| PDE4 only | 13 | 14 | 14,523 | –0.25 (–0.92 to 0.42) | 0.78 (0.4 to 1.52) | .463 | 0.00 |
| Non-BD | 57 | 91 | 56,715 | –0.1 (–0.19 to –0.01) | 0.91 (0.83 to 0.99) | .036 | 0.06 |
| ≥ 1 Exacerbation in previous year | 27 | 47 | 31,055 | –0.07 (–0.17 to 0.04) | 0.93 (0.84 to 1.04) | .204 | 0.00 |
For this analysis, the model is ln(RR) = a + b*dTreat, where RR = rate ratio. The unit for dTreat unit is 100 mL. See Table 1 legend for expansion of abbreviations.
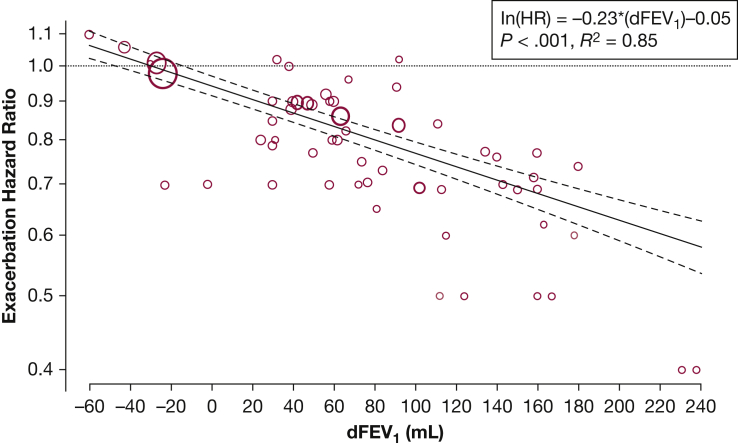
We also evaluated the effect of dFEV1 on the HR for moderate to severe exacerbations (Fig 4). Compared with the RD and RR analyses, there were significantly fewer trials that performed HR analyses. For the broadest comparison, an improvement in predose FEV1 of 100 mL was associated with a 21% reduction in the risk of moderate to severe exacerbation (HR, 0.79; 95% CI, 0.74-0.83; P < .001; R2 = 0.85). For an improvement of 50 mL, there was an 11% reduction in risk (HR, 0.89; 95% CI, 0.87-0.91, P < .001). This association was demonstrated in all subgroups, except for the PDE4 inhibitors (Table 3).
Figure 4.
Effect of dFEV1 on exacerbation HR. Each comparison is represented by a circle, and the regression line is in solid black with 95% CIs shown. The y-axis is the natural logarithm of the HR. The unit for dFEV1 in the regression equation is 100 mL. HR = hazard ratio. See Figure 2 legend for expansion of other abbreviation.
Table 3.
Effect of dFEV1 on the HR for Exacerbation
| Inclusion | No. of Trials | No. of Comparisons | No. of Subjects | Slope (b) Estimate (95% CI) |
HR = Exp(b) Estimate (95% CI) |
P | R2 |
|---|---|---|---|---|---|---|---|
| All studies | 39 | 65 | 73,475 | –0.23 (–0.28 to –0.18) | 0.79 (0.74 to 0.83) | < .001 | 0.85 |
| Only two arms | 19 | 19 | 27,578 | –0.27 (–0.43 to –0.12) | 0.74 (0.64 to 0.86) | < .001 | 0.65 |
| BD only | 20 | 34 | 53,308 | –0.25 (–0.32 to –0.19) | 0.77 (0.72 to 0.82) | < .001 | 0.88 |
| ICS only | 12 | 17 | 18,415 | –0.21 (–0.37 to –0.04) | 0.81 (0.69 to 0.96) | .013 | 0.63 |
| PDE4 only | 6 | 6 | 7,349 | 0.06 (–1.28 to 1.41) | 1.06 (0.28 to 4.11) | .926 | 0.00 |
| Non-BD | 22 | 31 | 27,750 | –0.17 (–0.28 to –0.07) | 0.84 (0.76 to 0.93) | < .001 | 0.68 |
| ≥ 1 Exacerbation in previous year | 13 | 21 | 19,663 | –0.26 (–0.37 to –0.15) | 0.77 (0.69 to 0.86) | < .001 | 0.75 |
For this analysis, our model is ln(HR) = a + b*dTreat, where HR = hazard ratio. The unit for dTreat is 100 mL. See Table 1 legend for expansion of other abbreviations.
Lastly, we identified potential outlier studies and recalculated the meta-regression equations with these studies excluded. The slope, intercept, and R2 estimates were not significantly different (data not shown).
Before inclusion of the moderator variable, all of the analyses demonstrated significant statistical heterogeneity. The I2 statistic for the RD, RR, and HR analyses was 90%, 85%, and 78%, respectively. After inclusion of dFEV1 as the moderator variable, the residual I2 statistics were 88%, 81%, and 33%.
Discussion
This comprehensive analysis illustrated the relationship between reduced COPD exacerbation risk and improvements in FEV1 resulting from various therapeutic interventions in the context of randomized controlled clinical trials. This association is consistent with previous studies that showed an association between lower FEV1 and increased exacerbation risk.12, 13 In addition, we generated mathematical equations to predict changes in exacerbation risk and rates according to dFEV1.
There are few prospective studies that both demonstrate COPD exacerbation risk reduction and at the same time explore the potential underlying mechanism. The most relevant study, which evaluated changes in inflammatory markers with tiotropium, failed to note any difference despite improvements in FEV1 and reductions in COPD exacerbations.14 Given the paucity of mechanistic studies and because our analysis only demonstrates an association, we are limited to speculation about the underlying etiology of the association we found.
There are several potential biologic pathways that may explain why improved airway patency is associated with reductions in COPD exacerbations. Greater airway patency might lead to better clearance of secretions, with consequent reductions in bacterial colonization and thus reductions in exacerbations. Sethi15 and Veeramachaneni and Sethi16 have proposed that exacerbations of COPD are related to lower airway bacterial colonization, and several previous studies have shown that lower airway bacterial colonization leads to local inflammation17, 18, 19, 20, 21 and is associated with increased exacerbation risk.18 Acquisition of new bacterial strains may portend an exacerbation of COPD,22 but studies examining the association of bacterial load and exacerbations have produced mixed results.23, 24 There do not appear to be any previous studies that have examined both the change in airway patency and alterations in bacterial colonization.
Improved airway patency might work to reduce exacerbations through other mechanisms, such as improved symptom control. For example, improvements in airway patency are associated with reductions in dyspnea and increased physical activity.25 This link is likely due to increased resting inspiratory capacity and decreased dynamic hyperinflation during exercise.26 Improved symptoms could lead to less frequent reporting of exacerbations, or greater physical activity itself may result in fewer COPD exacerbations. Although one study reported an association between physical activity and lower rates of COPD exacerbations,27 another did not.28
The present RD and RR analyses showed that a small amount of heterogeneity is explained by the change in FEV1; however, we did not find this association for the subgroup of all non-BD trials. Thus, it remains reasonable to conclude that some COPD medications might have a direct antiinflammatory effect in the airways that is the primary driver of exacerbation risk reduction. In this scenario, dFEV1 might be functioning either as a surrogate biomarker of effectiveness or as an integral part of the mechanistic pathway. Whether dFEV1 is an epiphenomenon or plays a direct role in the reduction of COPD exacerbations needs to be examined in future studies.
The present study highlights the statistical challenges of measuring COPD exacerbation frequency and risk. Depending on the exacerbation measure used, our results demonstrate substantial differences in the amount of between-study variance attributable to dFEV1. We found that dFEV1 explained most of the variance in HRs but did not account for as much of the variance in RDs and RRs.
There are several potential residual sources of statistical heterogeneity. Absolute exacerbation rates are dependent on patient characteristics, trial design, and statistical methods. A previous study reported significant between-study variability in measurement and statistical analysis of COPD exacerbations.29 Some trials require patients to have an exacerbation in the previous year, and these trials will have higher exacerbation rates. In addition, a trial may or may not follow up with patients after they drop out and include their exacerbations, or they might require patients to drop out after their first exacerbation. Significant dropout may lead to a “healthy survivor” effect that skews the measurement of exacerbation rates and can significantly change the exacerbation rate.30 More recent trials use Poisson and negative binomial regression analyses, which can model the loss to follow-up and thus may more accurately estimate an exacerbation rate.31
HR analysis circumvents many of the aforementioned issues. Because the first exacerbation is the outcome, the HR bypasses the questions regarding multiple exacerbations. Patient dropout is factored into the analysis and thus does not affect the ratio (as long as proportional hazards are maintained). Lastly, because it provides no estimates of the incidence of exacerbations, it is not influenced by trial entry criteria, reducing between-study variability. These factors likely all contribute to the differences between the HR analysis and the RD and RR analyses. For reasons articulated, studies examining COPD exacerbation risk reduction should always include HR for time to first exacerbation as a prespecified end point.
Our study has several strengths. We included a broad range of studies across multiple therapeutic classes and demonstrated that improving predose FEV1 is robustly associated with exacerbation risk reduction. Because between 39 and 94 trials were included in these analyses, the results were not dependent on a few, large trials. We also included several unpublished studies, identified from Cochrane meta-analyses and pharmaceutical company clinical trial online databases, thus reducing the risk that publication bias influenced our results.32, 33, 34
There are several limitations to our study. First, we did not contact study authors for missing data, and thus our results may be subject to this aspect of publication bias. However, whenever possible, we did use unpublished sources, such as clinicaltrials.gov results and company trial registries, to fill in the missing data. In addition, with a large number of trials and patients included, it is unlikely that the addition of a few more trials will substantially alter our results. Second, we had to impute SEs for RDs and RR because they were often not reported. This assumption may be faulty if the underlying distributions were not normal. Third, there was variability in precision of dFEV1 reporting, and thus trials with precision of only 100 mL for dFEV1 may have detracted significantly from our ability to infer a correlation. Fourth, for our RD and RR analyses, we also were unable to control for differences in baseline characteristics due to the variability in reporting across multiple studies. Fifth, we did not include nonpharmacologic interventions, and thus our results are not applicable to other treatments for COPD, such as physical rehabilitation, vaccines, or alternative and complementary medicine. Lastly, we did not systematically assess the potential biases of studies included in the analysis.
Conclusions
This meta-regression analysis revealed a robust correlation between the reduction in risk of COPD exacerbations and therapeutic improvements in lung function, supporting the hypothesis that improvement in airway patency is an important mechanism contributing to the reduction in exacerbation risk. Future studies regarding the mechanism of reduction of COPD exacerbation risk could investigate changes in bacterial colonization and rates of acquisition of new bacteria, to further elucidate possible mechanistic steps between improvements in airway patency and reduction in exacerbation risk.
Acknowledgments
Author contributions: A. D. Z. takes responsibility for (is the guarantor of) the content of the manuscript, including the data and analysis. A. D. Z., X. W., R. G. B., W. S., I. Z. B., and C. B. C. contributed substantially to the study design, data analysis and interpretation, and the writing of the manuscript.
Financial/nonfinancial disclosures: The authors have reported to CHEST the following: A. D. Z. reports that his spouse is an employee and stockholder of Shire. W. S. reports that she has received grants and/or personal fees from Boehringer Ingelheim, Novartis, AstraZeneca, and Chiesi Farmaceutici S.p.A, none in relation to this work. I. Z. B. reports that he has received personal fees from AstraZeneca and Grifols, none in relation to this work. C. B. C. reports that he has received grants and/or personal fees from Amgen, Spiration, PulmonX, Boehringer Ingelheim, GlaxoSmithKline, and Equinox health clubs, none in relation to this work; in addition, he is currently employed as a global medical expert in the GlaxoSmithKline respiratory franchise. None declared (R. G. B., X. W.).
Role of sponsors: The sponsors had no role in the design of the study, the collection and analysis of the data, or the preparation of the manuscript.
Additional information: The e-Appendix, e-Figure, and e-Tables can be found in the Supplemental Materials section of the online article.
Footnotes
Portions of this research have been presented previously in abstract form at the 2016 American Thoracic Society Annual Meeting, May 13-18, 2016, San Francisco, CA.
FUNDING/SUPPORT: This study was funded by the UCLA Exercise Physiology Research Laboratory unrestricted funding. In addition, R. G. B. is supported by the National Institutes of Health/National Center for Advancing Translational Science UCLA CTSI [Grant Number TL1TR001883].
Supplementary Data
References
- 1.Jenkins C.R., Jones P.W., Calverley P.M. Efficacy of salmeterol/fluticasone propionate by GOLD stage of chronic obstructive pulmonary disease: analysis from the randomised, placebo-controlled TORCH study. Respir Res. 2009;10:59. doi: 10.1186/1465-9921-10-59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Blasi F., Cesana G., Conti S. The clinical and economic impact of exacerbations of chronic obstructive pulmonary disease: a cohort of hospitalized patients. PLoS One. 2014;9(6):e101228. doi: 10.1371/journal.pone.0101228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Seemungal T.A., Donaldson G.C., Paul E.A., Bestall J.C., Jeffries D.J., Wedzicha J.A. Effect of exacerbation on quality of life in patients with chronic obstructive pulmonary disease. Am J Respir Crit Care Med. 1998;157(5 pt 1):1418–1422. doi: 10.1164/ajrccm.157.5.9709032. [DOI] [PubMed] [Google Scholar]
- 4.Connors A.F., Jr., Dawson N.V., Thomas C. Outcomes following acute exacerbation of severe chronic obstructive lung disease. The SUPPORT investigators (Study to Understand Prognoses and Preferences for Outcomes and Risks of Treatments) Am J Respir Crit Care Med. 1996;154(4 pt 1):959–967. doi: 10.1164/ajrccm.154.4.8887592. [DOI] [PubMed] [Google Scholar]
- 5.Donaldson G.C., Seemungal T.A., Bhowmik A., Wedzicha J.A. Relationship between exacerbation frequency and lung function decline in chronic obstructive pulmonary disease. Thorax. 2002;57(10):847–852. doi: 10.1136/thorax.57.10.847. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Wouters E.F. Economic analysis of the Confronting COPD survey: an overview of results. Respir Med. 2003;97(Suppl C):S3–S14. doi: 10.1016/s0954-6111(03)80020-3. [DOI] [PubMed] [Google Scholar]
- 7.Global Strategy for Diagnosis, Management and Prevention of COPD. Global Initiative for Chronic Obstructive Lung Disease (GOLD), 2016. http://goldcopd.org. Accessed January 18, 2016.
- 8.van Houwelingen H.C., Arends L.R., Stijnen T. Advanced methods in meta-analysis: multivariate approach and meta-regression. Stat Med. 2002;21(4):589–624. doi: 10.1002/sim.1040. [DOI] [PubMed] [Google Scholar]
- 9.Raudenbush S. Analyzing effect sizes: random effects models. In: Cooper H., Hedges L.V., Valentine J.C., editors. The Handbook of Research Synthesis and Meta-Analysis. 2nd ed. Russell Sage Foundation; New York, NY: 2009. pp. 295–315. [Google Scholar]
- 10.Berkey C.S., Hoaglin D.C., Mosteller F., Colditz G.A. A random-effects regression model for meta-analysis. Stat Med. 1995;14(4):395–411. doi: 10.1002/sim.4780140406. [DOI] [PubMed] [Google Scholar]
- 11.R Core Team. R: a language and environment for statistical computing. Version 3.2.2. R Foundation for Statistical Computing, Vienna, Austria; 2015. https://www.R-project.org. Accessed August 31, 2015.
- 12.Niewoehner D.E., Lokhnygina Y., Rice K. Risk indexes for exacerbations and hospitalizations due to COPD. Chest. 2007;131(1):20–28. doi: 10.1378/chest.06-1316. [DOI] [PubMed] [Google Scholar]
- 13.Hoogendoorn M., Feenstra T.L., Hoogenveen R.T., Al M., Molken M.R. Association between lung function and exacerbation frequency in patients with COPD. Int J Chron Obstruct Pulmon Dis. 2010;5:435–444. doi: 10.2147/COPD.S13826. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Powrie D.J., Wilkinson T.M., Donaldson G.C. Effect of tiotropium on sputum and serum inflammatory markers and exacerbations in COPD. Eur Respir J. 2007;30(3):472–478. doi: 10.1183/09031936.00023907. [DOI] [PubMed] [Google Scholar]
- 15.Sethi S. Infection as a comorbidity of COPD. Eur Respir J. 2010;35(6):1209–1215. doi: 10.1183/09031936.00081409. [DOI] [PubMed] [Google Scholar]
- 16.Veeramachaneni S.B., Sethi S. Pathogenesis of bacterial exacerbations of COPD. COPD. 2006;3(2):109–115. doi: 10.1080/15412550600651347. [DOI] [PubMed] [Google Scholar]
- 17.Sethi S., Maloney J., Grove L., Wrona C., Berenson C.S. Airway inflammation and bronchial bacterial colonization in chronic obstructive pulmonary disease. Am J Respir Crit Care Med. 2006;173(9):991–998. doi: 10.1164/rccm.200509-1525OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Patel I.S., Seemungal T.A., Wilks M., Lloyd-Owen S.J., Donaldson G.C., Wedzicha J.A. Relationship between bacterial colonisation and the frequency, character, and severity of COPD exacerbations. Thorax. 2002;57(9):759–764. doi: 10.1136/thorax.57.9.759. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Wilkinson T.M., Patel I.S., Wilks M., Donaldson G.C., Wedzicha J.A. Airway bacterial load and FEV1 decline in patients with chronic obstructive pulmonary disease. Am J Respir Crit Care Med. 2003;167(8):1090–1095. doi: 10.1164/rccm.200210-1179OC. [DOI] [PubMed] [Google Scholar]
- 20.Soler N., Ewig S., Torres A., Filella X., Gonzalez J., Zaubet A. Airway inflammation and bronchial microbial patterns in patients with stable chronic obstructive pulmonary disease. Eur Respir J. 1999;14(5):1015–1022. doi: 10.1183/09031936.99.14510159. [DOI] [PubMed] [Google Scholar]
- 21.Hill A., Campbell E., Hill S., Bayley D., Stockley R. Association between airway bacterial load and markers of airway inflammation in patients with stable chronic bronchitis. Am J Med. 2000;109(4):288–295. doi: 10.1016/s0002-9343(00)00507-6. [DOI] [PubMed] [Google Scholar]
- 22.Sethi S., Evans N., Grant B.J., Murphy T.F. New strains of bacteria and exacerbations of chronic obstructive pulmonary disease. N Engl J Med. 2002;347(7):465–471. doi: 10.1056/NEJMoa012561. [DOI] [PubMed] [Google Scholar]
- 23.Sethi S., Sethi R., Eschberger K. Airway bacterial concentrations and exacerbations of chronic obstructive pulmonary disease. Am J Respir Crit Care Med. 2007;176(4):356–361. doi: 10.1164/rccm.200703-417OC. [DOI] [PubMed] [Google Scholar]
- 24.Wilkinson T.M., Hurst J.R., Perera W.R., Wilks M., Donaldson G.C., Wedzicha J.A. Effect of interactions between lower airway bacterial and rhinoviral infection in exacerbations of COPD. Chest. 2006;129(2):317–324. doi: 10.1378/chest.129.2.317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Jones P.W., Donohue J.F., Nedelman J., Pascoe S., Pinault G., Lassen C. Correlating changes in lung function with patient outcomes in chronic obstructive pulmonary disease: a pooled analysis. Respir Res. 2011;12:161. doi: 10.1186/1465-9921-12-161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.O'Donnell D.E., Webb K.A. The major limitation to exercise performance in COPD is dynamic hyperinflation. J Appl Physiol (1985) 2008;105(2):753–755. doi: 10.1152/japplphysiol.90336.2008b. discussion 755-757. [DOI] [PubMed] [Google Scholar]
- 27.Esteban C., Arostegui I., Aburto M. Influence of changes in physical activity on frequency of hospitalization in chronic obstructive pulmonary disease. Respirology. 2014;19(3):330–338. doi: 10.1111/resp.12239. [DOI] [PubMed] [Google Scholar]
- 28.Schönmann M., Sievi N.A., Clarenbach C.F. Physical activity and the frequency of acute exacerbations in patients with chronic obstructive pulmonary disease. Lung. 2015;193(1):63–70. doi: 10.1007/s00408-014-9673-7. [DOI] [PubMed] [Google Scholar]
- 29.Aaron S.D., Fergusson D., Marks G.B. Counting, analysing and reporting exacerbations of COPD in randomised controlled trials. Thorax. 2008;63(2):122–128. doi: 10.1136/thx.2007.082636. [DOI] [PubMed] [Google Scholar]
- 30.Suissa S. Statistical treatment of exacerbations in therapeutic trials of chronic obstructive pulmonary disease. Am J Respir Crit Care Med. 2006;173(8):842–846. doi: 10.1164/rccm.200508-1338PP. [DOI] [PubMed] [Google Scholar]
- 31.Keene O., Jones M., Lane P., Anderson J. Analysis of exacerbation rates in asthma and chronic obstructive pulmonary disease: example from the TRISTAN study. Pharmaceutical statistics. 2007;6(2):89–97. doi: 10.1002/pst.250. [DOI] [PubMed] [Google Scholar]
- 32.Kew K.M., Dias S., Cates C.J. Long-acting inhaled therapy (beta-agonists, anticholinergics and steroids) for COPD: a network meta-analysis. Cochrane Database Syst Rev. 2014;(3):CD010844. doi: 10.1002/14651858.CD010844.pub2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Chong J., Leung B., Poole P. Phosphodiesterase 4 inhibitors for chronic obstructive pulmonary disease. Cochrane Database Syst Rev. 2013;(11):CD002309. doi: 10.1002/14651858.CD002309.pub4. [DOI] [PubMed] [Google Scholar]
- 34.Geake J.B., Dabscheck E.J., Wood-Baker R., Cates C.J. Indacaterol, a once-daily beta2-agonist, versus twice-daily beta(2)-agonists or placebo for chronic obstructive pulmonary disease. Cochrane Database Syst Rev. 2015;(1):CD010139. doi: 10.1002/14651858.CD010139.pub2. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.