Graphical abstract
Keywords: Type-2 diabetes mellitus, C2C12 cultured cell line, STZ induced diabetic rats, PTP-1B
Abstract
Background
To investigate the antidiabetic effect of Himalayan Medicinal plants from India viz. Melia azedarach (Family: Meliaceae), Zanthoxylum alatum (Family: Rutaceae), Tanacetum nubigenum (Family: Asteraceae) using in-vitro as well as in-vivo approaches.
Methods
Their effects were examined on stimulation of glucose uptake by C2C12 cultured cell line, inhibitory effect on human recombinant Protein tyrosine phosphatase-1B (PTP-1B) and followed by the hypoglycaemic activity of extracts in Streptozotocin (STZ) induced diabetic rats.
Results
All prepared extracts had been found to enrich with polyphenolic, flavonoids, terpenoids, anthraquinones and saponins type of compounds. n-Butanol fraction of Zanthoxylum alatum showed maximum PTP-1B inhibition (61.9%) whereas ethanol extract of Tanacetum nubigenum showed strong stimulation of glucose uptake (+61.2%) in C2Cl2 myotubes. In STZ induced Sprague-Dawley rats, significant decrease in blood glucose level was observed in ethanol extract of Melia azaderach treated group as 14.8% (p < 0.01) whereas in the ethanol extract of Tanacetum nubigenum treated group, it was observed as 15.5% (p < 0.01) compare to metformin which showed 26.8% (p < 0.01) lowering of blood glucose in the same time duration of 5 h study.
Conclusion
This study demonstrated that these plants have a significant therapeutic value in type-2-diabetes mellitus and related complications thus supporting their traditional uses in Indian traditional system of medicine.
1. Introduction
Type-2 diabetes mellitus (T2DM), a chronic and heterogeneous disease with diverse disorders, affect the metabolism of proteins, fat, and carbohydrates etc1, 2. Most prominent factors such as dyslipidemia, hyperglycemia, insulin resistance/impaired secretion and activation of proinflammatory mediators contribute rapid incidence and advancement of T2DM3 and thus it is considered as one of the most recurrent lifestyle diseases4. T2DM is a non-communicable disease with leading causes of death worldwide due to associated long term side effects include ketoacidosis, hyperosmolarcoma accompanied with chronic disorders, retinopathy, renal failure, neuropathy, skin complications, as well as increasing cardiovascular risks5, 6, 7, 8. In fact, T2DM affects more than 200 million people worldwide which are expected to reach 642 million by 2024 based on a report presented by International Diabetes Federation (IDF) in 20159, 10. Several hypoglycemic agents in the form of chemical formulations and synthetic compounds such as sulphonyl ureas, metformin and many others have been identified to provide a better treatment of T2DM but none of them have been found as a true antidiabetic agent without having any side effects11, 12, 13. Medicinal plants/herbs/foods are good sources of alternative or complementary medicines for the treatment of various diseases14, 15, 16. Based on World Health Organization (WHO) report, it is considered as approximately 75-80% of the world's population primarily depends on traditional formulations or medicine obtained from plant materials and products17. Herbal medicines are commonly prescribed throughout the world because of low side effects, easy availability, and reasonable cost and effectiveness parameters18, 19. In present study, three medicinal plants i.e., Melia azedarach (Family: Meliaceae), Zanthoxylum alatum (Family: Rutaceae), Tanacetum nubigenum (Family: Asteraceae) were taken to evaluate antidiabetic activity. These medicinal plants have been used in traditional systems of Indian medicine for treatment of various types of diseases and disorders.
Melia azedarach L., (MA) commonly known as Pride of India or Indian Lilac, is usually cultivated in Himalayan region of India, Pakistan, China, Burma, Bangladesh etc.20. The therapeutic uses of this plant are well documented in Ayurveda, Siddha and Unani System of Medicine21. Based on these systems, the rootbark &fruit are considered as astringent, tonic, antiperiodic, purgative, emollient and anthelmintic22, 23. The stembark is bitter, tonic, astringent, antiperiodic, vermifuge24. Leaf has discutient and anthelmintic action25. Seed Oil is stimulant, insecticide and antiseptic26. Many scientific literatures have been reported the various biological activities of MA including anticancer, antimalarial activity, analgesic, anti-inflammatory, anti-anthelmintic, antilithic, diuretic27, astringent and stomachic properties28, 29, 30. Zanthoxylum allatum, (ZA) commonly called as winged prickly ash or Timur or Toothache tree, is a small, aromatic and horny tree grows up to 3.5 meters in height and mainly found in Himalayan region of India, China, Japan, and Korea31, 32. It is used as an effective Ayurvedic herb for toothache treatment along with other numerous health benefits33. ZA has many traditional values to cure various body ailments and disorders related to digestion, circulation, immunity and skin. Fruit extracts are known to combat fever, dental problems, worms and dyspepsia because of antiseptic, deodorant and disinfectant properties. The bark of the tree is used for gums relief, asthma, diabetes and for snake bite34. On the basis of literature survey, various pharmacological activities of ZA in different parts have been reported as anti-inflammatory35, hepatoprotective36, 37, larvicidal38, antispasmodic, antidiarrheal, bronchodialator and in cardiovascular disorders39, antidysentric40, piscicide41, lousicidal potential42, cytotoxics43. Various part of ZA showed the presence of different types of active chemical constituents include essential oils, fatty acids, monoterpenes like linalool, limonene, flavonoids, flavonoids glycosides, volatile compounds like alkaloids, sterols, fatty acids, lignins, amino acids, aromatic compounds etc44, 45, 46, 47, 48. Tanacetum nubigenum DC (TN) (family: Asteraceae) is a small snow melts plant that wildy grows on rocky slopes, sandy ground in the 3600-4300 m elevation of the Kumaun and North-west Garhwal regions of the India49. TN is pleasant-smelling aromatic herb and thus used in preparation of incense and fragrant materials by the local inhabitant and in traditional medicines since ancient times. From this species, various pharmacological studies are reported as antifungal, anthelmintic, carminative, stimulant, antispasmodic, repellant and fumigant properties50, 51. Literature survey reveals that it has many types of compounds including essential oils, monoterpenes, sesquiterpenes and higher terpenoids49. In this article, we described the effect of ethanol extracts and fractions on PTP-1B enzyme inhibition as well as glucose uptake stimulation by C2C12 myotubes. Most active fractions were also assayed in STZ induced Sprague-Dawley rats model.
2. Methods
2.1. Chemical and Reagents
Streptozotocin, antibiotics (penicillin G, streptomycin, gentamycin, amphoterecin B), phosphate buffered saline, trypsin, Ethylene diamine tetra acetic acid (EDTA), insulin, were purchased from Sigma-Aldrich Chemical Company, St. Louis, USA. Metformin (standard drug) was a gift sample from Wokhardt Pvt. Ltd. Commercial reagents which are used in this study were of analytical grade. All solvents were distilled off prior use for extraction and fractionation process.
2.2. Plant materials
Plant materials were collected from the tropical, temperate and alpine zones of Garhwal region. Twigs of MA were collected from Srinagar. Leaves of ZA collected from Rudraprayag and leaves of TN were collected from Kedarnath. All plant samples were authenticated by a taxonomist of the Botany Department of HNB Garhwal University Uttarakhand. A voucher specimen for future correspondence is deposited in the Department of Botany, HNB Garhwal University, Uttarakhand (India) in 2013 (Table 1)
Table 1.
Profile of selected plant material
| S. No. | Plant species | Family | Part used | Abbreviation | Percentage yield (w/w) |
|---|---|---|---|---|---|
| 1 | Melia azadarach | Meliaceae | Twigs | MAT | 18.00 |
| 2 | Zanthoxylum alatum | Rutaceae | Leaves | ZAL | 21.50 |
| 3 | Tanacetum nubigenum | Asteraceae | Leaves | TNL | 23.15 |
MAT: Melia Azadarach Twigs; ZAL: Zanthoxylum Elatum Leaves; TNL: Tanacetum Nubigenum Leaves
2.3. Animals
Animals were procured from the animal colony of CSIR-Central Drug Research Institute, Lucknow, UP India. About 3-4 week old male Sprague Dawley rats with body weight 160 ± 20 g were procured.The present study was approved before start of experiment by the Animal Ethics Committee, Government of India constituted in 1964. All the rats were kept in animal housing facility by maintaining the standard conditions of optimum temperature (22 °C), relative humidity and a 12 h light/dark cycle with free access to diet and water unless stated otherwise. All rats were acclimatized for 4 days in laboratory conditions before start of experiment.
2.4. Preparation of ethanol extracts and fractions
After collection of plant materials, it was washed and dried at room temperature for 7 days and finely powdered in a grinding machine for ease of extraction. Ethanol as solvent was selected for extraction of plant material. Sample powders of each plant were soaked in ethanol (5.0 L) in a percolator. Percolation process was repeated up to three to five times till the plant material was extracted completely. The total extracts was collected, filtered and concentrated under vacuum using rotavapor at 40-450C and weighed. Crude ethanol extract was dissolved in distilled water (500 ml) and then successively partitioned (5 times each) with n-hexane, chloroform, and n-butanol in separating funnel. Each fraction was collected, dried, and stored at 4 °C and named as hexane, chloroform and n-butanol fractions respectively. Aqueous fraction (water soluble) was also concentrated under vacuum at 50-600C.
2.5. Cell culture of C2C12 myotubes
C2C12 myotubes were cultured in DMEM containing 10% fetal bovine serum (FBS), penicillin(120 units/ml), streptomycin (75 μg/ml), gentamycin (160 μg/ml) and amphotericin B (3 μg/ml). Cells were incubated in CO2Incubator in humid environment with 5% CO2. On 70% confluency the cell media was replaced from 10% FBS containing DMEM media with 2% FBS containing DMEM media for cellular differentiation and cells were kept for another 4-6 days in incubator.
2.6. Measurement of 2-deoxy-D-[1-3H] glucose
C2C12 myotubeswere grown in 24 well culture plates as described above. After differentiation cells were subjected to glucose uptake with some modification as reported in earlier studies52. In Brief C2C12 myotubes were incubated with 10 and 20 μg/ml concentration of plant extracts/fractions for 24 h after that 4 h in serum deprived medium further a sub-set of cells were stimulated with 100 nM insulin for 20 min. Glucose uptake was assessed for 5 min in HEPES- buffered saline [140 mMNaCl, 20 mM HEPES, 5 Mm KCl, 2.5 mM MgSO4, 1 mM CaCl2 (pH 7.4)] containing 10 μM 2-DG (0.5 μCi/ml 2-[3H] DG) at room temperature. On completion of glucose uptake cells were rinsed with an ice-cold 0.9% NaCl solution containing 20 mM D-glucose. After washing cells were lysed with 0.05N NaOH and lysates were counted with scintillation fluid in a β-counter to calculate radioactivity. Nonspecific uptake values were subtracted from all other values determined in the presence of cytochalasin B (50 μM).All the reactions were done in triplicate and normalized to total protein. Glucose uptake values were expressed as fold induction with respect to unstimulated cells.
2.7. Protein Tyrosine Phosphatase-1B (PTP-1B) inhibition assay
PTP-1B inhibition assay was done using colorimetric, nonradioactive PTP-1B drug discovery kit (BML-AK 822, Enzo Life Sciences, USA) according to the manufacturer instructions53. In brief plant fractions were incubated with human recombinant PTP-1B enzyme at different concentration in 96 well flat bottomed microtiter plates. After adding the substrate to each well Plates were incubated at 30 °C for 30 min. On termination of reaction released phosphate was determined by the addition of 25 μl of biomol red reagent. The final concentration of DMSO used to dissolve the plant fractions in the test well (1.0%) had no demonstrable effect on PTP-1B enzyme activity. All the plant fractions were tested at five different concentrations as 10, 20, 30, 50 and 100 μM (Table 1, Table 2). PTP-1B phosphatase acts on the phosphopeptide substrate and releases phosphate group. The activity of PTP-1B was measured in terms of released free phosphate. After adding biomol red to reaction wells, plate was kept for next 20 min to develop the color. On completion of incubation absorbance was measured at 620 nm on a microplate reader. PTP-1B enzyme inhibition was calculated as percent inhibition of PTP1B activity in the control tube (without inhibitor) as 100%. Formula used to calculate activity was as-% Inhibition= (OD in control well –OD in Test well)/OD in control well*100
Table 2.
Effect of selected plant extracts and fractions on PTP-1B and glucose uptake C2C12 muscle cells
| S. No. | Sample code | Extract/fractions |
% Stimulation on Glucose uptake |
% Inhibition of PTP-1B activity |
|||
|---|---|---|---|---|---|---|---|
| 10 μg/ml | 20 μg/ml | 10 μg/ml | 20 μg/ml | 50 μg/ml | |||
| 1 | MATE | Ethanol extract | +19.7 | +23.6 | 28.9 | 30.1 | 42.5 |
| 2 | MATH | Hexane fraction | +9.5 | +12.7 | 45.8 | 51.7 | 56.9 |
| 3 | MATC | Chloroform fraction | +11.2 | +14.8 | 37.9 | 44.1 | 49.4 |
| 4 | MATB | Butanol fraction | +23.4 | ND | 57.6 | 72.0 | 68.5 |
| 5 | ZALE | Ethanol extract | Nil | +8.4 | 47.8 | 45.7 | 56.8 |
| 6 | ZALH | Hexane fraction | +6.3 | +9.1 | 41.9 | 48.5 | 56.9 |
| 7 | ZELC | Chloroform fraction | +9.2 | +14.7 | 49.2 | 56.7 | 59.2 |
| 8 | ZALB | Butanol fraction | +17.8 | ND | 61.9 | 70.9 | 71.5 |
| 9 | TNLE | Ethanol extract | +61.2 | +41.9 | 60.7 | 71.3 | 70.1 |
| 10 | TNLH | Hexane fraction | +8.7 | +17.3 | 40.3 | 48.8 | 56.2 |
| 11 | TNLC | Chloroform fraction | +13.2 | +15.8 | 53.4 | 58.1 | 66.2 |
| 12 | TNLB | Butanol fraction | +41.2 | ND | 59.6 | 63.8 | 64.4 |
Ethanol extract (E); Hexane fraction (H); Chloroform fraction (C); Butanol fraction (B); MATE: Melia Azadarach Twigs Ethanol; MATH: Melia Azadarach Twigs Hexane; MATC: Melia Azadarach Twigs Chloroform; MATB: Melia Azadarach Twigs Butanol; ZALE: Zanthoxylum Elatum Leaves Ethanol; ZALH: Zanthoxylum Elatum Leaves Hexane; ZALC: Zanthoxylum Elatum Leaves Chloroform; ZALB: Zanthoxylum Elatum Leaves Butanol; TNLE: Tanacetum Nubigenum Leaves Ethanol; TNLH: Tanacetum Nubigenum Leaves Hexane; TNLC: Tanacetum Nubigenum Leaves Chloroform; TNLB: Tanacetum Nubigenum Leaves Butanol
2.8. Streptozotocin (STZ)-induced diabetes
STZ was dissolved in Citrate buffer (0.1 M) and injected intraperitoneally in overnight fasted 80 Sprague-Dawley albino rats of body weight 160 ± 20 g at the dose of 60 mg/kg body weight. After 48 h of injection fasting blood glucose level of animals was measured from cut tail vain using glucometer (Accu-Chek). Animals showing fasting blood glucose level between 300-450 mg/dl were considered as diabetic and further divided into desired experimental groups containing 8 animals in per group. Each group received oral doses of fine, suspension of test samples prepared in 1% gum acacia at a dose of 250 mg/kg and 100 mg/kg for extracts and fractions respectively. Metformin was used as standard antidiabetic drugs at a dose of 100 mg/kg body weight. During experiments blood of animals were measured at time interval of 30, 60, 90, 120, 180, 240, 300 min and at 1440 min post test sample/standard drug or vehicle treatment. To determine the percentage blood glucose lowering by test substance or standard drug Blood glucose level (mg/dl) vs time (min) were plotted on Graph Pad Prism. Area under the curve (AUC) between 0-300 min and 0-1440 min was calculated from the graph and by comparing the AUC of test substance treated/standard drug treated groups to show the improvement in hyperglycemia.
2.9. Cell Cytotoxicity assay
The cytotoxicity of samples was evaluated in MTT assay on RAW 264.7 (mouse macrophage cells). The RAW 264.7 cells were seeded onto a 96-well plate at a density of 1.0 × 104 cells per well in DMEM containing 10%Fetal Bovine serum and incubated at 37oC. At approximately 70% of confluence cells were treated with various compounds at different concentrations from 200 μg/ml to 12.5 μg/ml. After additional 24 h incubation at 37o C, 100 μL of MTT (0.5 mg/mL in PBS) was added to the wells and incubated for another 3 h. After incubation cell media was removed and cells were fixed by adding methanol to each well. Then after removing the methanol 200 μL DMSO was added to each well to solubilize the color compound. The resulting color was assayed at 540 nm using a micro plate spectrophotometer (Molecular Devices). Cell viability is proportion to color intensity of MTT assay. Results are calculated by using following formula as-
2.10. Statistical analysis
Data was analyzed on Graph Pad Prism 5 software and the results are expressed as mean ± SD. Unpaired Student's t-test was used for analyzing the data between two groups and one-way ANOVA followed by Dunnett test (used for multiple comparisons) was used for more than two groups Values were considered statistically significant at p < 0.05, p < 0.001 and p < 0.0001.
3. Results
3.1. Effect of crude extracts and their fractions on glucose uptake by C2Cl2 cells
The present study shows that ethanol extract of MA twigs (MATE), butanol extract of MA twigs (MATB), butanol extract of ZA leaves (ZALB), ethanol extract of TN leaves (TNLE) and butanol extract of TN leaves (TNLB) increase the glucose uptake by 19.7%, 23.4%, 17.8%, 61.2% and 41.2% respectively at 10 μg/ml concentration as shown in Table 2.
3.2. Effect of crude extracts and their fractions on PTP-1B activity
The effect of crude extracts and fractions were studied for PTP-1B inhibitory activity at five different concentrations, i.e. 10, 20, 30, 50 and 100 μg/ml concentrations. In this paper, we are reporting inhibitory activity only at three concentrations 10, 20 and 30 μg/ml which are summarized in Table 2. MATB, ZALB, TNLE, TNLB and chloroform fraction of TN leaves (TNLC) exhibited significant inhibition of PTP-1B at 57.6%, 61.9%, 60.7%, 59.6% and 53.4% inhibition, respectively at 10 μg/ml concentration, whereas at 20 μg/ml concentration, they showed significant inhibition of 72.0%, 70.9%, 71.3.3%, 58.1% and 57.7% inhibition respectively (Table 2).
3.3. The effect of ethanol extracts on the blood glucose level of STZ induced Sprague-Dawley rats
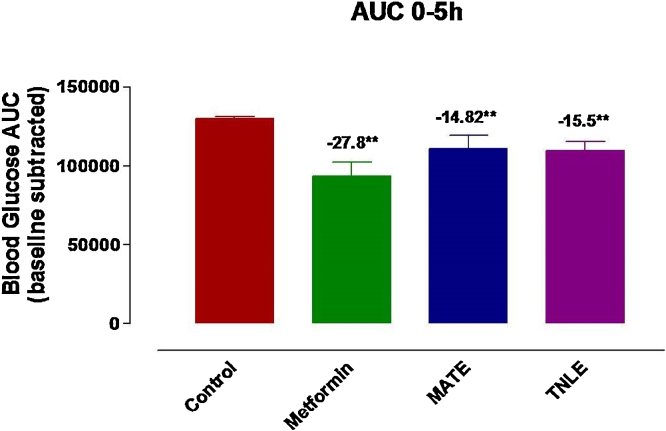
A pool of β-cell destruction in STZ-induced diabetic rats results into severe insulin deficiency followed by elevation in fasting blood glucose level beyond the normal value. All the animals were treated with ethanol extracts as listed in Table 1. Results show moderate to low antihyperglycemic activity in blood glucose AUC in single dose animal experiments except significant lowering with ethanolic extract of MA and TN. The most significant effect as hyperglycemia was found only in MATE tune of 14.8% (p < 0.01) whereas in TNLE it was observed in 15.5% (p < 0.01) during 5 h study. Moreover, metformin (standard drug) caused 27.8% (p < 0.01) lowering of blood glucose in the same time of experiment. After 24 hours of the experiment decrease in hyperglycemia was found only in MATE tune of 15.8% (p < 0.01) whereas in TNLE it was observed in 10.8% (p < 0.05) whereas metformin lowered down 26.8% (p < 0.01) blood glucose in similar manner (Fig. 1, Fig. 2, Fig. 3).
Fig. 1.
Anti hyperglycaemic activity of ethanol extract of Melia azaderach twigs (MATE) and ethanol extract of Tanacetum nubigenum leaves (TNLE) on STZ-induced diabetic rats at the dose of 250 mg/kg).
Fig. 2.
AUC (Area under curve) values showing the effect of ethanol fraction of Melia azaderach twigs (MATE), ethanol extract of Tanacetum nubigenum leaves (TNLE) on 18 h fasted STZ-induced diabetic rats over a period of 5 h comparing with the standard anti-diabetic drugs (metformin). AUC values are presented as mean ± SD and significance was calculated as comparison to control group. ** p < 0.001.
Fig. 3.
Effect of butanol fraction of Melia azaderach twigs (MATB), butanol extract of Zanthoxylum alatum leaves (ZELB) and Tanacetum nubigenum leaves (TNLB) on 18 h fasted STZ-induced diabetic rats over a period of 24 h comparing with the standard anti-diabetic drugs (metformin).
3.4. Effect of n-butanol, chloroform and hexane fractions on the blood glucose level of STZ induced Sprague-Dawley rats
The effect of various plant fractions was tested for their antihyperglycemic activity in a single dose experiment on STZ induced diabetic rats as stated in experimental design. It is evident from the results that when diabetic rats were treated with plant fractions MATB, ZALB and TNLB. All treated groups were also observed for lowering in blood glucose level, among them most significant decrease in hyperglycemia was found only in MATB and TNLB samples which were observed as 17.9% (p < 0.01) and 21.3% (p < 0.01) respectively during 5 h study. After 24 hours of the experiment MATB treated animals showed a decrease of 20.8% and TNLB treated animals showed 22.1%, respectively whereas metformin was found to show 26.8% (p < 0.01) lowering of blood glucose in the same manner (Fig. 4, Fig. 5, Fig. 6).
Fig. 4.
Anti hyperglycaemic activity of Melia azaderach twigs (MATB), butanol fraction of Zanthoxylum alatum leaves (ZELB) and butanol fraction of Tanacetum nubigenum leaves (TNLB) on STZ-induced diabetic rats at the dose of 100 mg/kg shown by AUC. Values are presented as mean ± SD and significance was calculated as comparison to control group. *p < 0.05,** p < 0.001.
Fig. 5.
Effect of butanol fraction of Melia azaderach twigs (MATB), butanol fraction of Tanacetum nubigenum leaves (TNLB) and butanol fraction of Zanthoxylum alatum leaves (ZELB) on 18 h fasted STZ-induced diabetic rats over a period of 5 h comparing with the standard anti-diabetic drugs (metformin) using two way analysis of variance (ANOVA) as analytical tool shown by AUC.AUC values are presented as mean ± SD and significance was calculated as comparison to control group. ** p < 0.001.
Fig. 6.
AUC values showing effect of butanol fraction of Melia azaderach twigs (MATB), butanol fraction of Zanthoxylum alatum leaves (ZELB), butanol fraction of Tanacetum nubigenum leaves (TNLB) on 18 h fasted STZ-induced diabetic rats over a period of 24 h comparing with the standard anti-diabetic drugs (metformin) using two way analysis of variance (ANOVA) as analytical tool.Values are presented as mean ± SD and significance was calculated as comparison to control group. ** p < 0.001.
3.5. Effect of extract and fractions on cell cytotoxicity
Additionally, the cytotoxicity profile of extracts and fractions was also evaluated in MTT assay on RAW 264.7 (mouse macrophage cells). All the extracts and fraction were found to have non toxic nature as shown in Table 3.
Table 3.
Cell viability (percent) using Raw 264.1 mouse macrophage
| S. No. | Sample code |
% cell viability at different concentration |
||||
|---|---|---|---|---|---|---|
| 200 μg/ml | 100 μg/ml | 50 μg/ml | 25 μg/ml | 12.5 μg/ml | ||
| 1. | MATE | 98.2 | 96.7 | 89.7 | 109.4 | 89.1 |
| 2. | MATB | 86.4 | 89.4 | 105.2 | 99.2 | 100.7 |
| 3. | ZALB | 110.5 | 96.5 | 102.8 | 93.6 | 107.1 |
| 4. | TNLE | 95.6 | 115.0 | 101.7 | 111.7 | 110.9 |
| 5. | TNLB | 99.1 | 97.4 | 91.7 | 105.7 | 101.0 |
4. Discussion
All prepared extracts and fractions were evaluated for their in vitro as well as in vivo hypoglycaemic activities via inhibition of PTP-1B enzyme activity, stimulation of glucose uptake by C2C12 as well as improvement in blood glucose profile in STZ induced Sprague-Dawley rats as compared to the standard metformin, a hypoglycaemic drug in order to validate their effect. Uses of medicinal plants for the treatment of various ailments have their own merits and demerits. Diabetes being a metabolic disorder bears a lot of complications if it is not properly managed. Unavailability of suitable antidiabetic agents and side effects associated with synthetic drug regimes prompt us to search out newer antidiabetic agents from natural resources. This study sketches out the antidiabetic potential of three medicinal plants from Himalyan region of India. Extensive literature survey and preliminary phytochemical analysis showed the presence of polyphenol, phenolic acid, flavonoids, triterpenoids, phytosterols and anthraquinones in different parts of these plants. Polyphenols including flavonoids are act as free radical scavengers to improve the life status in the diabetic patients by lowers down the chronic hyperglycemia and oxidative stress54. The antidiabetic activity in plants may be attributed to the presence of phenolic and flavonoid compounds. In our study, the butanol fractions of selected plant species generally have been found more active than other fractions, indicating that compounds having antidiabetic activity are polar in nature which established the significant correlations of polyphenolic and flavonoids to lower down the diabetic effect.
We have previously observed that isolated compounds from Melia azedarach leaves and fruits showed a potent antidiabetic activity. Ethanol extract prepared from Melia azedarach leaves and fruits showed 40.7 and 55.9% inhibition of PTP-1B enzyme at 10 μg/ml concentration. Furthermore, chloroform fraction of fruits and butanol fraction of leaves were found to inhibit PTP-1B enzyme by 50.2% and 65.5% at the same 10 μg/ml concentration, respectively55. Therefore this prompted us to study the different part of plants for anti-diabetic potential. To this, we selected the twig part of this plant and found significant results where MATE and its MATB fraction showed the inhibition of PTP-1B enzyme by 28.9% and 57.6% at 10 μg/ml concentration respectively. Additionally, they enhanced 19.7% and 23.7% stimulation of glucose uptake by C2Cl2 muscle cells. Leaves extract of ZA exhibited a weak reduction in blood glucose via inhibition of PTP-1B enzyme as well as glucose uptake stimulation. ZALB was found as significant active and inhibited in-vitro PTP-1B enzyme activity up to 57.7% at 10 μg/ml concentrations whereas it stimulated C2C12 muscle cells by 17.8% at 10 μg/ml concentration. There is no report was found on antidiabetic potential of this plant. Therefore it was also selected under this study. Ethanol extract of leaves of TN was found to stimulate the glucose uptake in C2Cl2 muscle cells by 61.2% at 10 μg/ml dose as well as it also inhibited the PTP-1B activity up to 60.7% at the same concentration. Among the tested fractions, TNLB was most active and enhance the 41.2% stimulation of glucose uptake in similar C2C12 muscle cells. It also showed the 59.0% inhibition of PTP-1B enzyme activity at 10 μg/ml concentration. Among all extracts and fractions, only MATE and TNLE samples reduce blood glucose in STZ-treated rats. MATE lowers down blood glucose by tune of 14.8% (p < 0.01) whereas MATB showed reduction of blood sugar in same rats up to 17.9% (p < 0.01) during 5 h study. In addition, TNLE, when administered orally in STZ-induced diabetic rats during 5 h study, produced 15.5% (p < 0.01) dose-dependent reduction of blood glucose. After 24 h of study, TNLE reduced only 10.8% (p < 0.05) blood glucose in similar animal model which was very low with compare to standard drug metformin (Fig. 1, Fig. 2, Fig. 3, Fig. 4, Fig. 5, Fig. 6).
In conclusion, our study revealed that the extract and fractions of selected plant species have the potential to inhibit PTP-1B activity and to stimulate the glucose uptake by C2C12 myotubes. Most active fractions viz MATE and TNLE exhibited significant lowering of blood sugar in STZ STZ induced Sprague-Dawley rats. Among them, Melia azedarach twigs and Tanacetum nubigenum leaves extract possess strong in-vitro as well as in-vivo antidiabetic effects which may be responsible for their hypoglycemic property. Furthermore, pharmacological and chemical investigations are under process to find out the active constituents responsible for antidiabetic activity and to elucidate its mode of action.
Conflict of interest
The authors declare no conflict of interest.
Acknowledgement
All authors are very grateful to CSIR, New Delhi through providing us funding for this work in the form of a major research project No. 02(0085)/12/EMR-II.
References
- 1.Bhowmik D., Tripathi K.K., Das B.C., Kumar K.P.S. Natural medicine used in the traditional Indian medical system for therapy of diabetes mellitus. Int J Chem Res. 2011;1:28–38. [Google Scholar]
- 2.Akash M.S.H., Shen Q., Rehman K., Chen S. Interleukin-1 receptor antagonist: a new therapy for type 2 diabetes mellitus. J Pharm Sci. 2012;101:1647–1658. doi: 10.1002/jps.23057. [DOI] [PubMed] [Google Scholar]
- 3.Akash M.S.H., Rehman K., Chen S. Role of inflammatory mechanism in pathogenesis of type 2 diabetes mellitus. J Cell Biochem. 2013;114:525–531. doi: 10.1002/jcb.24402. [DOI] [PubMed] [Google Scholar]
- 4.Mathur M.L., Gaura J., Sharma R., Haldiya K.R. Antidiabetic properties of a spice plant Nigella sativa. J Clin Endocrinol Metab. 2011;1:1–8. [Google Scholar]
- 5.Shaw C.S., Clark J., Wagenmakers J.M. Effect of exercise and nutrition on intramuscular fat metabolism and insulin sensitivity. Annu Rev Nutr. 2010;30:13–34. doi: 10.1146/annurev.nutr.012809.104817. [DOI] [PubMed] [Google Scholar]
- 6.Gleckman R., Morr J. Diabetes-related foot infections. Contemp Intern Med. 1994;6:57–64. [PubMed] [Google Scholar]
- 7.Behradmanesh S., Derees F., Rafieian-kopaei M. Effect of Salvia officinalis on diabetic patients. J Renal Injury Preven. 2013;2:51–54. doi: 10.12861/jrip.2013.18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Chakraborty U., Das H. Antidiabetic and antioxidant activities of Cinnamomum tamala leaf extracts in streptozotocin-treated diabetic rats. Glob J Biotech Biochem. 2010;5:12–18. [Google Scholar]
- 9.Singab A.N., Youssef F.S., Ashour M.L. Medicinal plants with potential antidiabetic activity and their assessment. Medicinal and Aromatic Plants. 2014;3:151–159. [Google Scholar]
- 10.International Diabetes Federation . sixth edition. International Diabetes Federation; Brussels, Belgium: 2014. IDF Diabetes Atlas. update. [Google Scholar]
- 11.Li W.L., Zheng H.C., Bukuru J., Kimpe N.D. Natural medicines used in the traditional Chinese medical system for therapy of diabetes mellitus. J Ethnopharmacol. 2004;92:1–21. doi: 10.1016/j.jep.2003.12.031. [DOI] [PubMed] [Google Scholar]
- 12.Nissen S.E., Wolski K. Effect of Rosiglitazone on the risk of myocardial infarction and death from cardiovascular causes. N Engl J Med. 2007;356:2457–2471. doi: 10.1056/NEJMoa072761. [DOI] [PubMed] [Google Scholar]
- 13.Nissen S.E. Setting the record straight. J Am Med Assoc. 2010;303:1194–1195. doi: 10.1001/jama.2010.333. [DOI] [PubMed] [Google Scholar]
- 14.Nasri H., Rafieian-Kopaei M. Herbal medicine and diabetic kidney disease. J Nephropharmacol. 2013;2:1–2. [PMC free article] [PubMed] [Google Scholar]
- 15.Eddouks M., Chattopadhyay D., De Feo V., Cho V.C. Medicinal plants in the prevention and treatment of chronic diseases. Evid Based Complement Alternat Med. 2014 doi: 10.1155/2014/180981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Jamila F., Mostafa E. Ethnobotanical survey of medicinal plants used by people in Oriental Morocco to manage various ailments. J Ethnopharmacol. 2014;154:76–87. doi: 10.1016/j.jep.2014.03.016. [DOI] [PubMed] [Google Scholar]
- 17.Singh R., Verma P.K., Singh G. Total phenolic, flavonoids and tannin contents in different extracts of Artemisia absinthium. J Intercult Ethnopharmacol. 2012;1:101–104. [Google Scholar]
- 18.Venkatesh S., Reddy G.D., Reddy B.M., Ramesh M., Rao A.V. Antihyperglycemic activity of Carallumaattenuate. Fitoterapia. 2003;74:274–279. doi: 10.1016/s0367-326x(03)00021-2. [DOI] [PubMed] [Google Scholar]
- 19.Nasri H., Shirzad H. Toxicity and safety of medicinal plants. J Herb Med Pharmacol. 2013;2:21–22. [Google Scholar]
- 20.Adnan Y., L-RubaeA The Potential Uses of Melia Azedarach L as Pesticidal and Medicinal Plant, Review. Am. -Eurasian J Sustain Agric. 2009;3:185–194. [Google Scholar]
- 21.Chopra R.N., Nayar S.L., Chopra I.C. Council of Scientific and Industrial Research; New Delhi: 1986. Glossary of Indian medicinal plants. [Google Scholar]
- 22.Cropley T.G., Haseqawa G.R. Melia azedarach: New potential for an old medicinal plant. J Am Acad Derma. 2007;52:366–367. doi: 10.1016/j.jaad.2007.04.013. [DOI] [PubMed] [Google Scholar]
- 23.Ahmad M., Khan M.A., Arshad M., Zafar M. Ethnophytotherapical approaches for the treatment of Diabetes by the local inhabitants of District Attock (Pakistan) Pak J Bot. 2011;43:141–150. [Google Scholar]
- 24.Rahmatullah M.M., Khatun A., Morshed N., Neogi P.K. A randomized survey of medicinal plants used by folk medicinal healers of sylhet division, Bangladesh. Adv Nat App Sci. 2010;4:52–62. [Google Scholar]
- 25.Husain S.Z., Malik R.N., Javaid M., Bibi S. Ethonobotanical properties and uses of medicinal plants of Morgah Biodiversity Park. Rawalpindi. Pak J Bot. 2008;40:1897–1911. [Google Scholar]
- 26.Sen A., Batra A., Rao D.V. Pivotal role of plant growth regulators in clonal propogation of Melia azedarach L. Int J Pharm Sci Rev Res. 2010;5:43–49. [Google Scholar]
- 27.Joy P.P., Thomas J., Mathew S., Skaria B.P. 1998. Medicinal Plants. Kerala Agriculture University. Aromatic and medicinal plants research station; pp. 193–194. [Google Scholar]
- 28.Andrade-Cetto A., Heinrich M. Mexican plants with hypoglycaemic effect used in the treatment of diabetes. J Ethnopharmacol. 2005;99:325–348. doi: 10.1016/j.jep.2005.04.019. [DOI] [PubMed] [Google Scholar]
- 29.Syamsudin Standardization of extract of Leucaena leucocephala (lmk) de Wit seeds by ?.?.-glucosidase inhibitor. Int J Phytomed. 2010;2:430–435. [Google Scholar]
- 30.Warrier P.K., Nambiar V.P.K., Ramankutty C. Orient Longman Ltd.; Hyderabad, India: 1995. Indian medicinal plants, a compendium of 500 species; pp. 10–12. [Google Scholar]
- 31.Singh T.P., Singh O.M. Phytochemical and pharmacological profile of Zanthoxylum armatum DC:An overview. Indian Journal of Natural Products and Resources. 2011;2:275–285. [Google Scholar]
- 32.Gupta S., Bhaskar S., Andola H.C. Altitudinal Variation in Essential Oil in Leaves of ZanthoxylumalatumRoxb. A High Value Aromatic Tree from Uttarakhand. J Med Plant Res. 2011;5:348–351. [Google Scholar]
- 33.Brijwal L., Pandey A., Tamta S. An overview on phytomedicinal approaches of Zanthoxylum armatumDC.: An important magical medicinal plant. J Med Plants Res. 2013;7:366–370. [Google Scholar]
- 34.Alam F., Us Saqib Q.N. Pharmacognostic study and development of quality control parameters for fruit bark and leaf of Zanthoxylum armatum (Rutaceae) AncSci Life. 2015;34:147–155. doi: 10.4103/0257-7941.157159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Bhatt N., Upadhyaya K. Anti-inflammatory activity of ethanolic extract of bark of Zanthoxylum armatum D.C. Pharmacology online. 2010;2:123–132. [Google Scholar]
- 36.Ranawat L., Bhatt J., Patel J. Hepatoprotective activity of ethanolic extracts of bark of Zanthoxylumarmatum DC in CCl4 induced hepatic damage in rats. J Ethnopharmacol. 2010;127:777–780. doi: 10.1016/j.jep.2009.10.019. [DOI] [PubMed] [Google Scholar]
- 37.Verma N., Khosa R.L. Hepatoprotective activity of leaves of Zanthoxylum armatum DC in CCl4 induced hepatotoxicity in rats. Indian J Biochem Biophys. 2010;47:124–127. [PubMed] [Google Scholar]
- 38.Tiwary M., Naika S.N., Tewary D.K., Mittal P.K., Yadav S. Chemical composition and larvicidal activities of the essential oil of Zanthoxylum armatum DC (Rutaceae) against three mosquito vectors. J Vect Born Dis. 2007;44:198–204. [PubMed] [Google Scholar]
- 39.Gilani S.N., Khan A.U., Gilani A.H. Pharmacological basis for the medicinal use of Zanthoxylum armatum in gut, airways and cardiovascular disorders. Phytother Res. 2010;24:553–558. doi: 10.1002/ptr.2979. [DOI] [PubMed] [Google Scholar]
- 40.Kar A., Borthakur S.K. Medicinal plants used against dysentery, diarrhea and cholera by the tribes of erstwhile Kameng district of Arunachal Pradesh. Nat Prod Rad. 2008;7:176–181. [Google Scholar]
- 41.Ramanujam S.N., Ratha B.K. Effect of alcohol extract of a natural piscicide -fruits of Zanthoxylum armatum DC on Mg2+ and Na+, K+ -ATPase activity in various tissues of a fresh water air-breathing fish, Heteropneustes fossilis. Aquaculture. 2008;283:77–82. [Google Scholar]
- 42.Kumar S., Singh S.K., Ghildiyal J.C., Baslas R.K., Saxena A.K. The lousicidal potential of the seed extract of Zanthoxylum alatum. Indian Vet J. 2003;80:848–850. [Google Scholar]
- 43.Barkatullah I.M., Muhammad N. Evaluation of Zanthoxylum armatum DC for in- vitro and in-vivo pharmacological screening. Afr J Pharm Pharmacol. 2011;5:1718–1723. [Google Scholar]
- 44.Ishii H., Ishikawa T., Chen I.S. Alkaloids of rutaceous plants. XXII. Cuspidiol, a new monomeric phenylpropanoid. Tetrahedron Lett. 1973;14:4189–4192. [Google Scholar]
- 45.Baneerjee H., Pal S., Adityachaudhury N. Occurrence of rutaecarpine in Zanthoxylum budrunga. Planta Med. 1989;55:403. doi: 10.1055/s-2006-962049. [DOI] [PubMed] [Google Scholar]
- 46.Chen I.S., Wu S.J., Tsai I.L. Chemical and bioactive constituents from Zanthoxylum simulans. J Nat Prod. 1994;57:1206–1211. doi: 10.1021/np50111a003. [DOI] [PubMed] [Google Scholar]
- 47.Chen I.S., Tsai I.W., Teng C.M., Chen J.J., Chang Y.L., Ko F.N., Lu M.C., Pezzuto J.M. Pyranoquinoline alkaloids from Zanthoxylum simulans. Phytochemistry. 1997;46:525–529. [Google Scholar]
- 48.Tsai I.L., Lin W.Y., Teng C.M., Ishikawa T., Doong S.L., Huang M.W. Coumarins and antiplatelet constituents from the root bark of Zanthoxylum schinifolium. Planta Med. 2000;66:618–623. doi: 10.1055/s-2000-8648. [DOI] [PubMed] [Google Scholar]
- 49.Beauchamp P., Dev V., Kashyap T., Melkani A., Mathela C., Bottini A.T. Composition of the Essential Oil of Tanacetum nubigenum Wallich ex DC. I Essent Oil Res. 2001;13:319–323. [Google Scholar]
- 50.Joshi R.K. Antifungal activity of Essential oil of Tanacetum longifolium Growing Wild in Uttrakhand, India. Journal of Biologically Active Products from Nature. 2013;3:97–100. [Google Scholar]
- 51.Haider S.Z., Mohan M., Pandey A.K., Singh P. Repellent and Fumigant Activities of Tanacetum nubigenum Wallich. ex DC Essential Oils against Tribolium castaneum (Herbst) (Coleoptera: Tenebrionidae) J Oleo Sci. 2015;64:895–903. doi: 10.5650/jos.ess15094. [DOI] [PubMed] [Google Scholar]
- 52.Ding H., Heng B., He W., Shi L., Lai C., Xiao L., Ren H., Mo S., Su Z. Chronic reactive oxygen species exposure inhibits glucose uptake and causes insulin resistance in C2C12 myotubes. Biochem Biophys Res Commun. 2016;478:798–803. doi: 10.1016/j.bbrc.2016.08.028. [DOI] [PubMed] [Google Scholar]
- 53.Joshi P., Deora G.S., Rathore V., Tanwar O., Rawat A.K., Srivastava A.K., Jain D. Identification of ZINC02765569: a potent inhibitor of PTP1B by vHTS. Med Chem Res. 2013;22:28–34. [Google Scholar]
- 54.Bahadoran Z., Mirmiran P., Azizi F. Dietary polyphenols as potential nutraceuticals in management of diabetes: a review. J Diabetes Metab Disord. 2013;12:43–51. doi: 10.1186/2251-6581-12-43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Chowtivannakul P., Srichaikul B., Talubmook C. Antidiabetic and antioxidant activities of seed extract from Leucaenaleucocephala (Lam.) de Wit. Agri Nat Resour. 2016;50:357–361. [Google Scholar]