Figure 5.
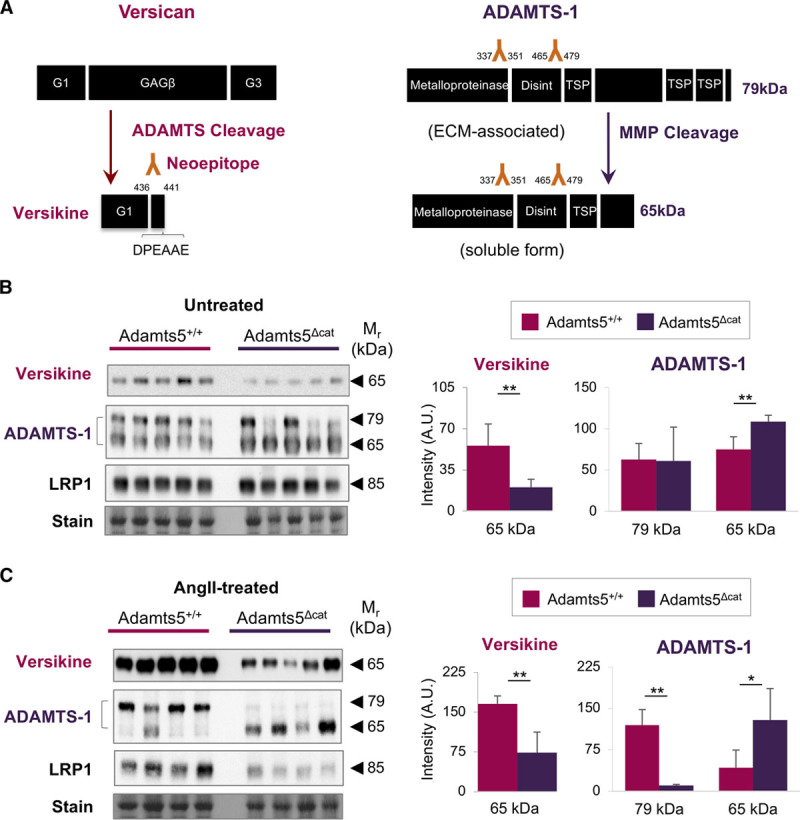
ADAMTS (a disintegrin and metalloproteinase with thrombospondin motifs)-1 cannot compensate for the lack of ADAMTS-5 activity. A, Left, Schematic representation of ADAMTS-mediated versican processing. The signature cleavage site for ADAMTS activity gives rise to an N-terminal versican fragment (versikine) and is recognized by a neoepitope antibody to the DPEAAE441 amino acid sequence. Right, Schematic representation of ADAMTS-1 processing. Processing by MMPs (matrix metalloproteinases) gives rise to a truncated, soluble form of ADAMTS-1 (65 kDa). B, C, Immunoblots using neoepitope antibodies to versikine were performed on aortic ECM (extracellular matrix) extracts from untreated and AngII (angiotensin II)-treated mice (n=5 per group). Immunoblots for ADAMTS-1 and LRP1 (low-density lipoprotein-related protein 1) were performed on total protein lysates of untreated and AngII-treated mice (n=5 and n=4 per group, respectively). Densitometry for the versican fragment (versikine) and ADAMTS-1. Bars represent mean±SD. Statistical significance was calculated using unpaired Student t tests. *P<0.05, **P<0.01. A.U. indicates arbitrary unit; and TSP, trombospondin domain.
