Fig. 1.
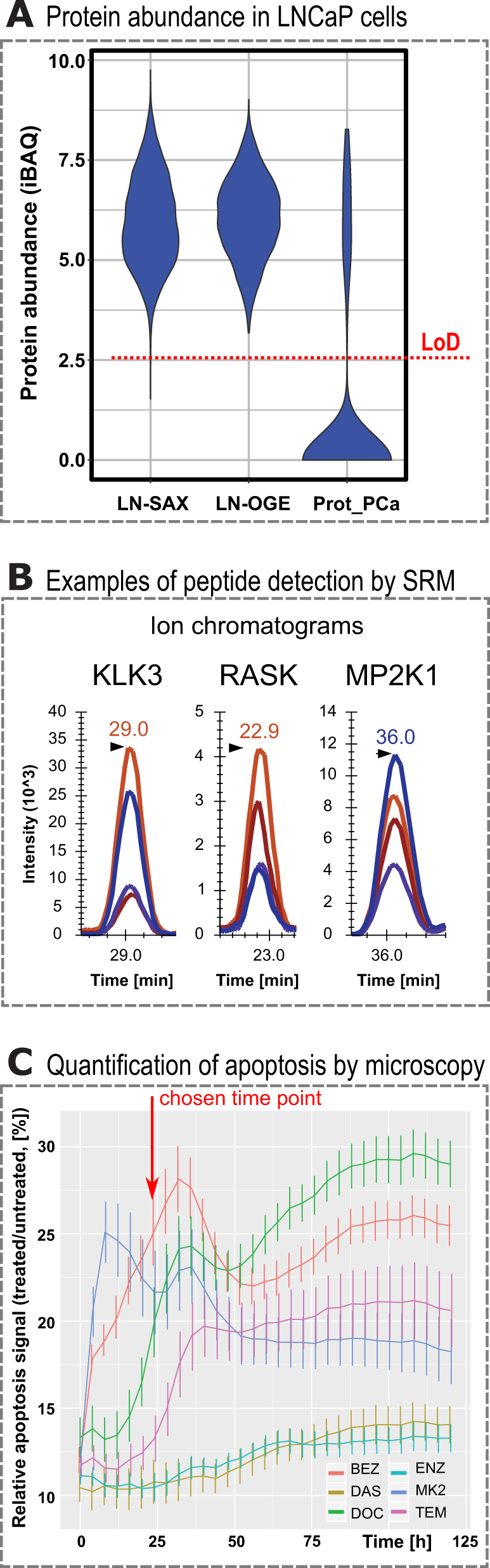
Development of SRM assays and initial drug testing. a Total proteome was isolated from LNCaP cells and fractionated using strong anion exchange (LN_SAE25). Separately, the LNCaP proteome was fractionated using strong cation exchange and off-gel electrophoresis (LN_OGE). In both cases, all fractions were analyzed using mass spectrometry (LC-MS/MS) and protein abundances calculated (iBAQ units64). The limit of detection (LoD) is indicated by a horizontal dashed red line. A quarter of the 490 proteins of interest in prostate cancer (Prot_PCa) are detectable in LNCaP cells using current mass spectrometry equipment. b For detectable proteins in LNCaP cell lysate SRM assays were developed. Examples of ion chromatograms show four co-eluting transitions with base line separation for peptides corresponding to KLK3 (prostate-specific antigen), RASK (GTPase KRas), and MP2K1 (MAP kinase kinase 1). c LNCaP cells were incubated with a caspase 3/7 fluorescent probe and six clinically relevant drugs (BEZ: NVP-Bez235, DAS: dasatinib, DOC: docetaxel, ENZ: enzalutamide, MK2: MK2206, TEM: temsirolimus). As a function of time the fluorescent signal corresponding to the degree of apoptosis was quantified by an automated fluorescent microscope. The ratio of treated to untreated apoptosis signal shows a large dynamic range at 24 h, a time point we chose for subsequent perturbation experiments
