Scheme 5. Application of the Strategy to C–H Alkenylation Reaction: (a) Electronic Effect of the Additive on the Selectivity and (b) C2-Selective C–H Alkenylation with Various Olefin Partners.
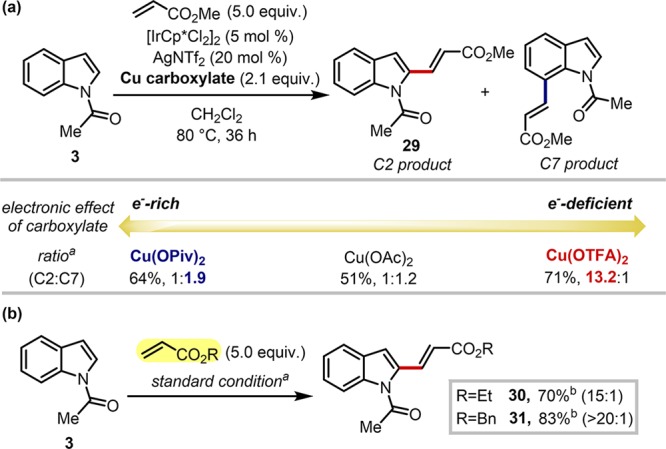
Reactions were run with 3 (0.20 mmol), acrylates (1.0 mmol), [IrCp*Cl2]2 (5 mol %), NaNTf2 (20 mol %), and copper carboxylate (0.42 mmol) in methylene chloride (1.5 mL) at 80 °C for 36 h. Site selectivities were determined by 1H NMR analysis of the crude mixture.
Isolated yields.
