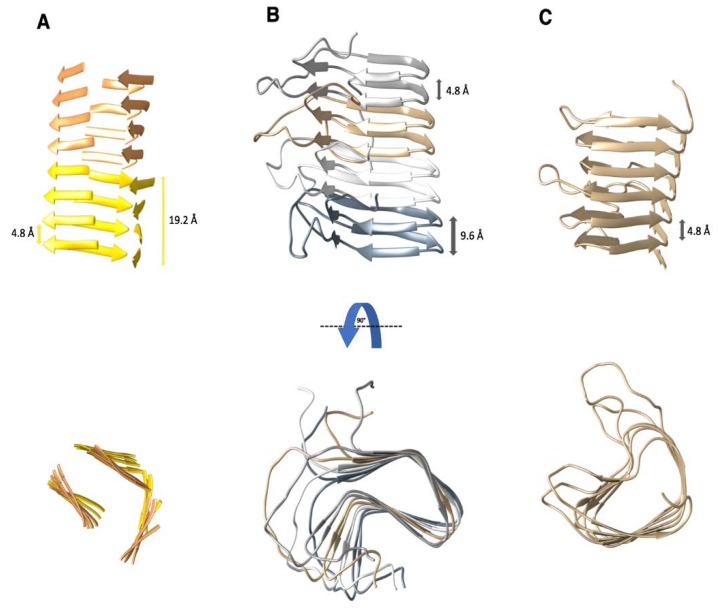
Figure 1.
Three examples of proteins that adopt a β-solenoid fold. The characteristic distance between individual β-rungs is 4.8 ± 0.2 Å. (A) Representative model of the 4-rung β-solenoid architecture of PrPSc based on X-ray fiber diffraction and cryo-EM [16,26]. Characteristic distances of the 4-rung β-solenoid spacing are labeled. Each cartoon color represents a single PrPSc monomer. (B) Structure of an amyloid fibril formed by the prion domain (residues 218–289) of the fungal prion HET-s. Each monomer adopted a 2-rung left-handed β-solenoid fold and is shown in a different color. PDB access code: 2rnm [31]. (C) Structure of the right-handed β-solenoid protein pectate lyase C from Erwinia chrysanthemi. Its N- and C-terminal caps were removed for representation of the β-solenoid structure (residues 118–285). PDB access code: 2pec [32].