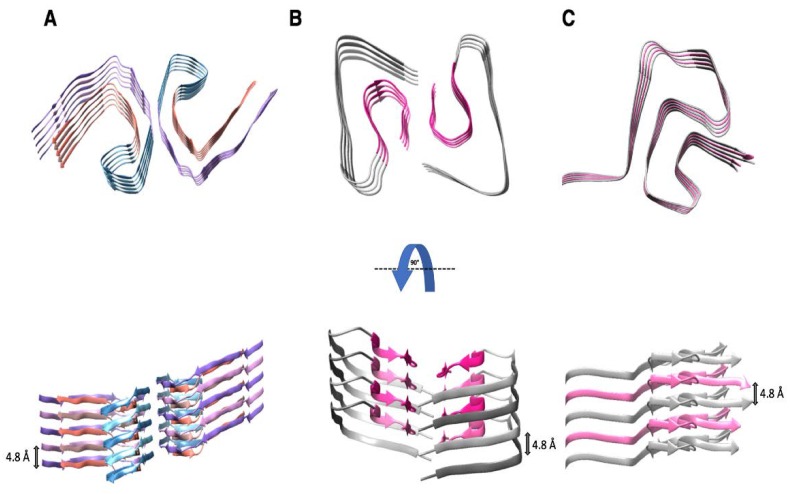
Figure 2.
Structures of pathogenic amyloid fibrils (A) paired helical filament (PHF) of tau; (B) Aß(1-42); and, (C) α-synuclein. The fibril cores of these proteins are arranged as parallel in-register ß-sheet structures, which are stabilized by hydrophobic interactions, salt bridges, and hydrogen bonds up and down the fibril axis. The axial distance between the stacked protein molecules is 4.8 ± 0.2 Å. (A) Top and the side views of a high-resolution structure of PHFs obtained by cryo-EM [20]. Five successive layers of the tau protein along the fibril axis revealed that the fibril core is composed of two C-shaped subunits (residues 306–378). PDB access code: 5o3l [20]. (B) Top and side views of a high-resolution structure of an Aß(1-42) fibril produced by cryo-EM [19]. The LS-shaped cross-sections of each protofilament reveal a staggered stacking of molecules along the fibril axis. PDB access code: 5oqv [19]. (C) Top and side views of a high-resolution structure of α-synuclein determined by ssNMR and X-ray fiber diffraction [21]. The fibril core of α -synuclein contains a Greek key motif based on a parallel in-register ß-sheet topology (residues 42–96). PDB access code: 2n0a [21].