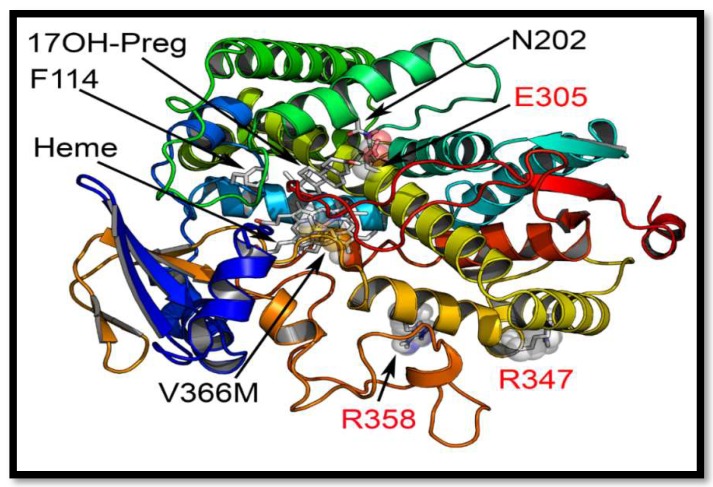
Figure 6.
Comparison of novel V366M mutation with the previously reported isolated CYP17A1-17,20 lyase mutations. Only three other residues in CYP17A1 have been reported to be mutated in patients with isolated 17,20 lyase deficiency. The R347 and R358 are at the redox partner binding sites and their mutations may interfere with binding of POR and /or cytochrome b5. The E305 residue at the active site is important for orientation of the substrate and its mutation has been shown to alter substrate specificity and lead to a preference for progesterone as the more efficient substrate. The V366 is located exactly at the active site of the CYP17A1.