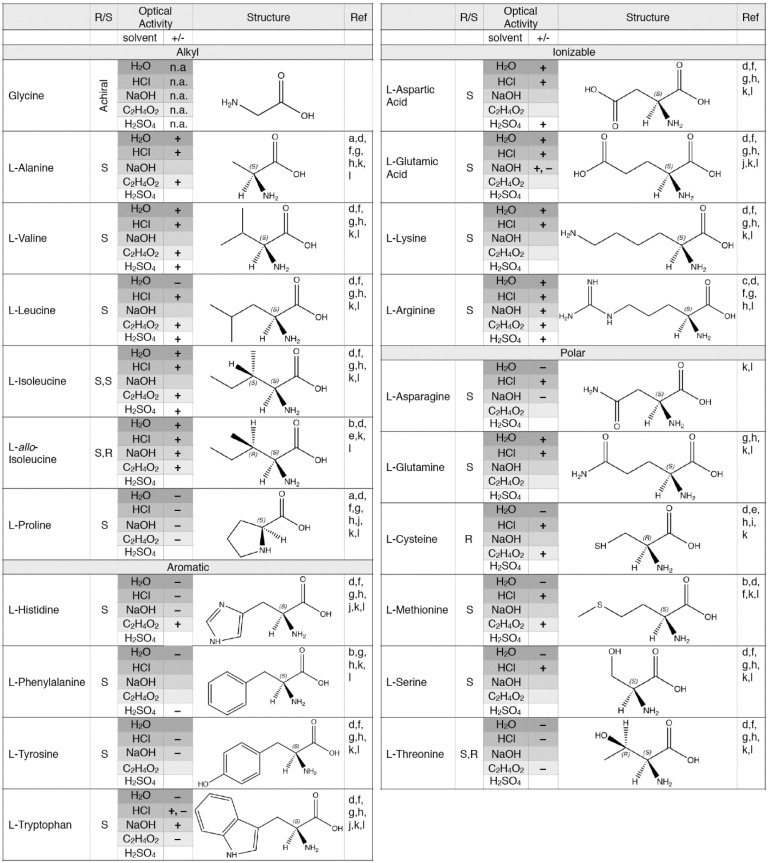
Figure 6.
The absolute configuration (R or S) of a chiral molecule is based on the arrangement of atoms directly attached to the stereocenter. For the l-enantiomers of the amino acids listed above, all but cysteine have an absolute configuration of S. The optical activity of chiral molecules describe the direction in which each enantiomer rotates plane polarized light; clockwise is dextrorotatory (+), counterclockwise direction is levorotatory (−). The sign of the optical rotation of an enantiomer is not directly related to its absolute configuration, and can vary based on the solution in which it is measured. In addition to being solution-dependent, the optical rotation varies with the molarity of each solution. For many of the amino acids, the entire range of specific rotation values fall in the positive or negative realm; exceptions to this include l-Glutamic Acid and l-Tryptophan, which have specific rotation values that vary between negative and positive numbers in specific solvents. l-cysteine, l-isoleucine, and l-allo-isoleucine are dextrorotatory in glacial acetic acid. References a-k correspond to [82,83,84,85,86,87,88,89,90,91,92,93].