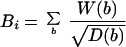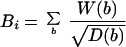Selection of variables that influence the distribution of centromeric α-satellite DNA. (a) G-banding ideograms exemplified for chromosomes 22 and 16 (
Francke, 1994), depicting D (linear genomic distance of the band center to the centromere), W (band width), a NOR band (the Nucleolar Organizer Region), a pericentromeric constitutive heterochromatin band (HET), and bands of staining intensity 0, 1, 2, 3 and 4 (G
0, G
1, G
2, G
3, and G
4). (b)Values for each variable. B
NOR takes value 1 for chromosomes containing a NOR and is zero otherwise. B
HET takes value 1 for chromosomes with an HET and is zero otherwise. GD indicates the gene density of each chromosome (genes/Mbp; from
Venter et al., 2001), and GC depicts the base composition of each chromosome (proportion of GC content as indicated by
Venter et al., 2001). The influence of bands of staining intensity
i is calculated for each chromosome as:
where
b is any band with staining intensity
i. In
Francke (1994), it is given the distance between the band borders and the centromere in units relative to chromosome arm length. We compute W(
b) and D(
b) from those values and from the genomic chromosome arm lengths given by
Morton (1991). (c) Statistics for the regression of α-satellite frequencies with each isolated variable. Statistically significant values are in italics. The slope of the linear regression is also indicated. A positive slope indicates that higher values of the variable correlate with a higher association with the specified nuclear compartment, whereas a negative slope indicates that lower values of the variable correlate with a higher association with the specified nuclear compartment. For example, the centromeres of chromosomes with a high GC content associate less with the nuclear lamina than centromeres of chromosomes with a low GC content.