Fig. 3. Innate immune responses in the lungs following trauma.
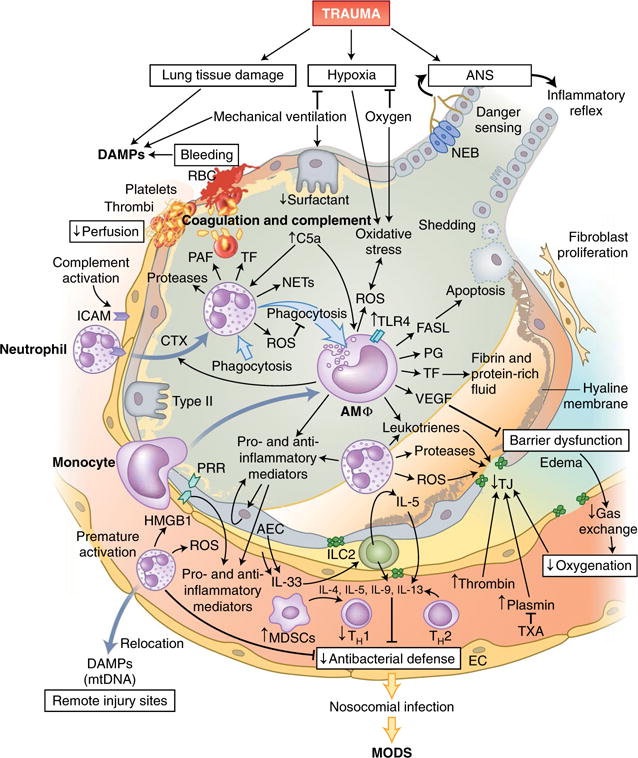
Tissue damage in the lungs and requisite mechanic ventilation lead to the release of large amounts of endogenous DAMPs, while bleeding and clot formation hinder the perfusion of alveolar capillaries. Leukocytes, primarily neutrophils and subsequently monocytes, which differentiate into macrophages on-site, invade the alveolar space via the activated endothelium and secrete inflammatory mediators and pro-coagulatory factors. Cellular debris and apoptotic neutrophils are removed by phagocytosis, which can induce a shift in macrophages from a pro-inflammatory phenotype to an anti-inflammatory phenotype. Alveolar macrophages (AMΦ) can also modulate the apoptosis of alveolar epithelial cells by secretion of the ligand for the death receptor Fas (FASL). Barrier degradation by neutrophil-derived proteases and ROS induces disruption of the air-blood-barrier, formation of edema in the extra-alveolar space, intra-alveolar accumulation of protein-rich fluids and the formation of hyaline membranes and thereby impairs gas exchange and blood oxygenation. Proteases of the coagulation cascade amplify the breakdown of TJs, which can be partially prevented by therapeutic application of the antifibrinolytic tranexamic acid (TXA). Furthermore, the local inflammatory response can be augmented by the recognition of DAMPs, including HMGB-1, by PRRs on alveolar endothelial and epithelial cells, which in turn release more DAMPs and mediators into alveoli and blood vessels. However, prematurely activated neutrophils are strongly attracted by other DAMPs, including mitochondrial DNA (mtDNA), and can relocate to remote injury sites and thus impair the local pulmonary antibacterial defense. IL-33 secreted by activated epithelial and endothelial lung cells activates group 2 ILCs (ILC2), which, via IL-5 production and a feed-forward loop, induce the secretion of more IL-5; this enhances a type 2 cytokine profile (IL-4, IL-5, IL-9 and IL-13) and further diminishes antibacterial potential. RBC, red blood cells; PAF, platelet-activating factor; Type II, type II alveolar epithelial cells; AEC, alveolar epithelial cells; NEB, neuroepithelial bodies; PG, prostaglandin; VEGF, vascular endothelial growth factor.
