Fig. 5. Trauma-induced breakdown of protective cell barriers in the gut.
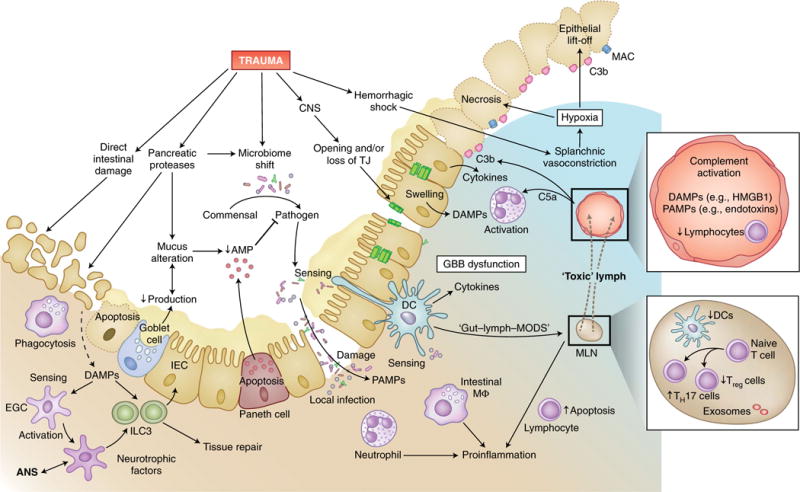
In addition to the direct tissue damage inflicted by abdominal injury, trauma can also indirectly induce dysfunction of the gut-blood barrier, with its central function of separating commensal and pathogenic intestinal bacteria from the predominantly sterile surrounding tissue and circulation. In intestinal tissue and blood vessels, trauma can induce lymphopenia, strong complement activation and large amounts of DAMPs and PAMPs, which in turn activate neutrophil granulocytes and recruit them to the intestine. Proteases increasingly secreted intraluminally from the pancreas in response to traumatic injury can alter the composition of mucus as well as its production in goblet cells and induce autodigestion of intestinal epithelial cells (IEC). Reduced secretion of host-defense peptides from Paneth cells leads to impaired detection and clearance of pathogenic bacteria, which can result in dysbiosis. Deteriorated barrier function and increased necrosis of intestinal epithelial cells, also due to the deposition of complement on damaged cells and hypoxia, as well as the loss of TJs (for example, after TBI), allow the transition of pathogens into the submucosal tissue. Local infection is sensed by neutrophils and resident intestinal macrophages, which induce a pro-inflammatory response and further damage the intestinal barrier. Intestinal DAMPs and PAMPs are screened by dendritic cells (DCs), which have spines that reach into the luminal space, as well as by enteric glial cells (EGC) that interact with neuronal cells of the ANS and group 3 ILCs (ILC3). Stimulated group 3 ILCs, in turn, promote intestinal tissue repair. Bacteria and their products can reach the lymph system either by direct translocation or, according to the ‘lymph-gut hypothesis’, by being transferred to mesenteric lymph nodes (MLN) by dendritic cells and intestinal macrophages, which results in the flow of ‘toxic’ lymph fluid into the venous system and presumably causes MODS. Furthermore, in the mesenteric lymph nodes, trauma can reduce the number of dendritic cells and thereby tip the TH17 cell/Treg cell balance toward a pro-inflammatory response. lympho, lymphocyte; AMP, antimicrobial peptide.
