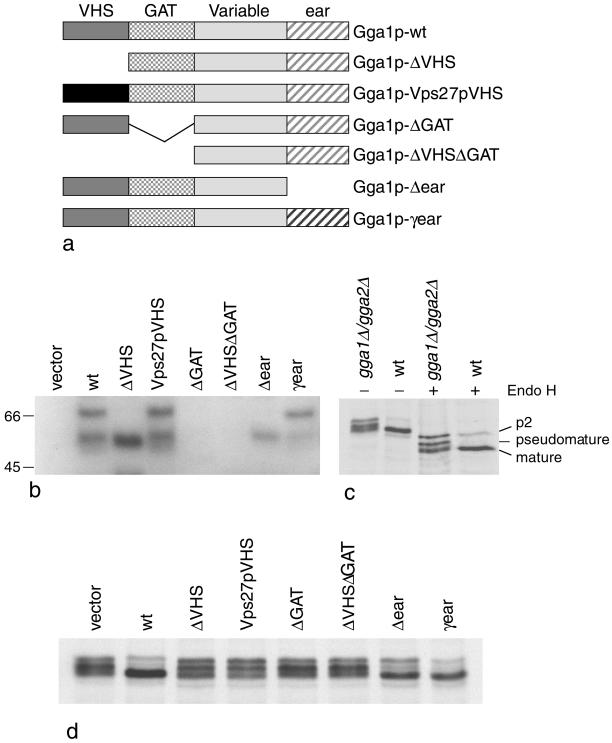
Figure 2.
(a) Diagrams of the Gga1p deletion mutants and chimeras. These constructs were all transformed into gga1Δ/gga2Δ cells. (b) Stability of the various constructs in yeast. Total extracts of cells expressing each of the constructs shown in a, as well as cells transformed with empty vector, were subjected to SDS-PAGE, loading equal amounts of protein in each lane, and Western blots were probed with anti-Gga1p. Labeled bands with the expected mobility are seen in most of the cells, with the exception of the cells transformed with the ΔGAT and ΔVHSΔGAT constructs, indicating that these constructs are unstable. The lower molecular weight band seen in some of the lanes presumably corresponds to a breakdown product. (c) CPY is aberrantly processed in gga1Δ/gga2Δ cells. Cells were labeled with 35S for 10 min and chased for 30 min, and then cell extracts were immunoprecipitated with anti-CPY. In the absence of endo H digestion, CPY in the gga1Δ/gga2Δ cells runs as a triplet. In the wild-type cells, most of the CPY comigrates with the lowest molecular weight band of the triplet, indicating that it has been processed to the mature form, although there is still a small amount of higher molecular weight (p2) CPY that has not yet been processed. In the presence of endo H, which removes N-linked oligosaccharides, the CPY in the gga1Δ/gga2Δ cells still runs as a triplet, indicating that the middle (pseudomature) band is the result of partial proteolysis rather than incomplete glycosylation. (d) CPY in cells expressing the constructs shown in a. The CPY in the cells transformed with empty vector runs as a triplet, whereas in the cells rescued with wild-type Gga1p most of the CPY runs at the mature position. The ΔVHS, ΔGAT, and ΔVHSΔGAT deletion mutants all fail to rescue the phenotype, although the mobility of the CPY is somewhat different in the cells expressing the ΔVHS construct. Unlike the other deletion mutants, the Δear mutant partially rescues the phenotype. The Vps27pVHS domain chimera does not rescue the phenotype; however, the γ-ear domain chimera appears to give full rescue.