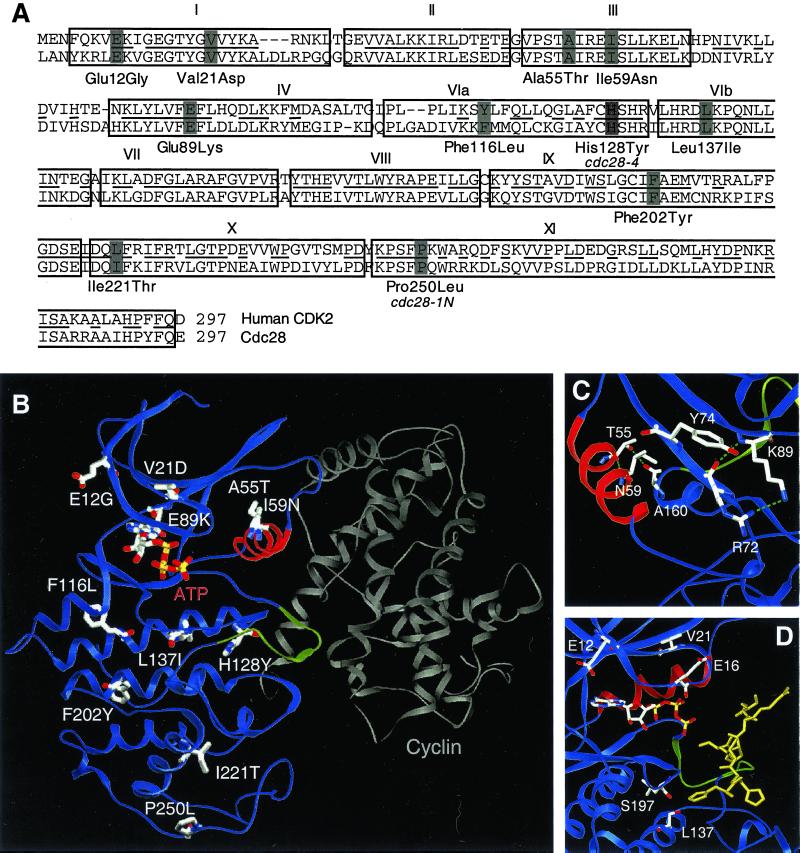
Figure 2.
Structural analysis of the cdc28ECP mutants. (A) Cdc28p and human CDK2 sequences aligned by Clustal (Higgins et al., 1996). Boxes delineate consensus kinase subdomains (Hanks et al., 1988) and bars decorate residues altered in cdc28-4, cdc28-1N, and the cdc28ECP mutants. (B) The residues altered in cdc28-4, cdc28-1N, and the cdc28ECP alleles are depicted on a ribbon diagram of Cdc28p modeled after CDK2 in active complex with cyclin A. The PSTAIRE helix is highlighted in red, the inhibitory T-loop in green, and cyclin A in gray. (C) Glu89Lys, Ala55Thr, and Ile59Asn, the mutations in or near PSTAIRE, are shown modeled into the inactive, monomeric form of the enzyme. The view shows the PSTAIRE helix (red) from the perspective of an “approaching” cyclin A. (D) The residues mutated in Leu137Ile, Glu12Gly, and Val21Asp are decorated in a model of Cdc28p with peptide substrate bound. The view shows the ATP as a stick diagram, PSTAIRE in red, the T loop in green, and the substrate peptide HHASPRK (Brown et al., 1999) in gold.