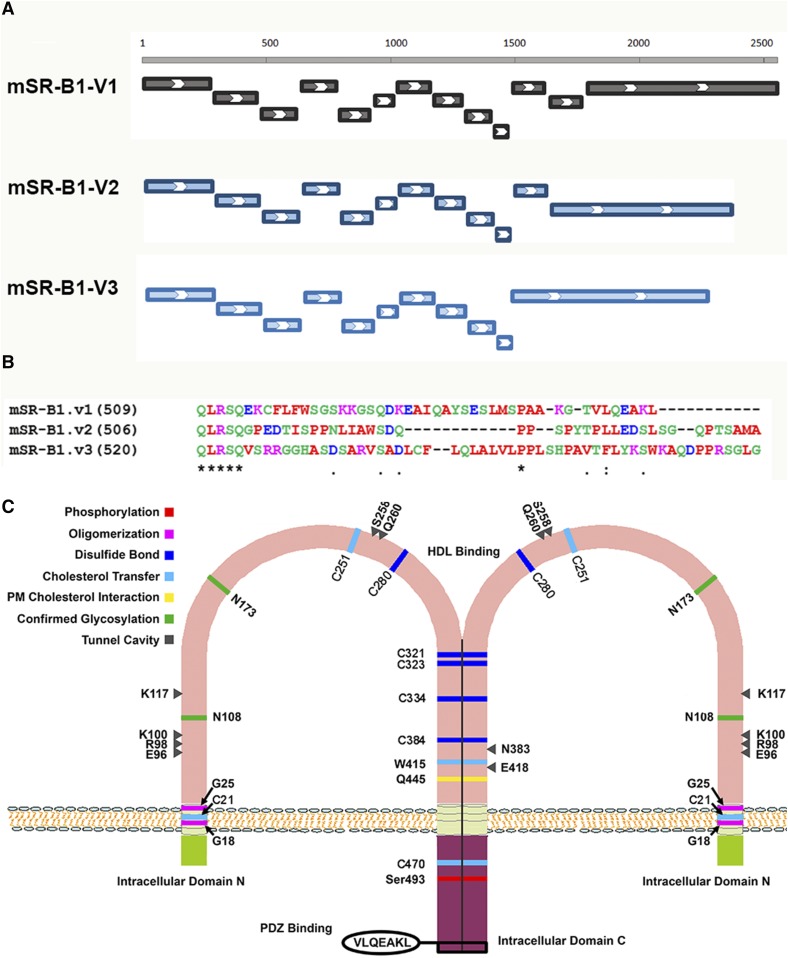
Fig. 1.
Gene exon organization (A) and C-termini sequences (B) of mouse SR-B1 variants and structural features of SR-B1 (C). A: Exon organization. Mouse SR-B1 variant 1 has 13 exons and 509 amino acids. Variant 2 arises from alternative splicing at the C-terminal end resulting in 12 exons and 506 amino acids. Genomic analysis shows that variant 3 has 11 exons and 520 amino acids. B: C-terminal sequences of mouse SR-B1 variants. C: Structural features of SR-B1. SR-B1 has an N-terminal and a C-terminal intracellular domain and an extended extracellular ectodomain. Several structural features are important for the function of SR-B1. An N-terminal transmembrane glycine motif (G15_G18_G25) was shown to be required for oligomerization and lipid transport. The C terminal of SR-B1 also contains sequences important for oligomerization. Six conserved cysteine residues (C251, C280, C321, C323, C334, and C384) found in the ectodomain of SR-B1 were also demonstrated to be involved in dimer/oligomer formation. Analysis of human SR-B1 revealed 11 putative N-linked glycosylation sites. Mutational analysis showed the importance of Asn-108 and Asn-173 for plasma membrane localization and for the ability to transfer lipid from HDL to cells. Structural analysis of SR-B1 homolog, LIMP-2, showed that there are eight amino acids that work coordinately to form a tunnel cavity that spans the entire length of the ectodomain allowing facilitated lipid transfer. E96, R98, K100, K117, W258, Q260, N383, and E418 are the amino acids that comprise the tunnel cavity to facilitate lipid transfer of SR-B1. The C-terminal intracellular domain contains an interacting domain (VLQEAKL) for binding with PDZ domain-containing proteins, through which the function of SR-B1 is regulated in a tissue-specific manner.