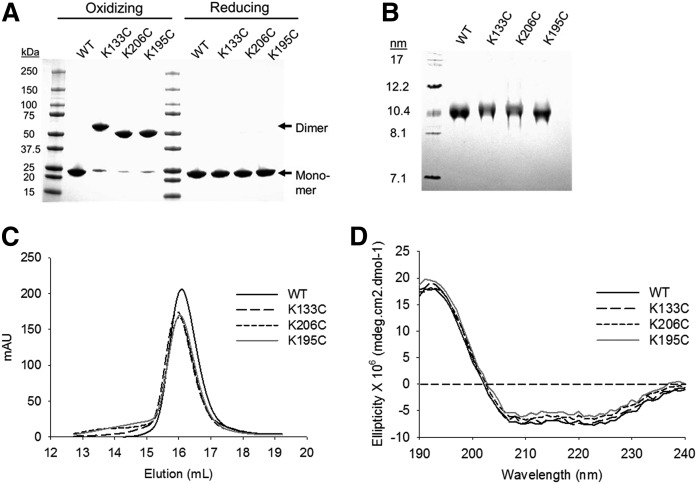
Fig. 3.
Characterization of Cys-mutant rHDL particles. A: Lipid-free WT APOA1 and Cys-mutants were predimerized in oxidizing conditions. Dimerized protein was then purified from the residual monomer by SEC. Protein purity was analyzed by SDS-PAGGE in oxidizing and reducing (3 mM β-ME) conditions. B: Sodium cholate dialysis was used to generate rHDL particles at a molar ratio of 80:8:1 POPC:cholesterol:APOA1. Native PAGGE was used to determine the purity of the particles in oxidizing conditions. C: SEC was used to determine the size and purity of the particles. Peak intensity is shown in arbitrary units (mAU) based on the elution volume of the particles. D: Far-UV CD spectra of WT and Cys-mutant rHDL particles showing the mean ellipticity of APOA1 based on the 1 nm wavelength increases in the UV spectrum (n ≥ 3 for all characterization experiments).