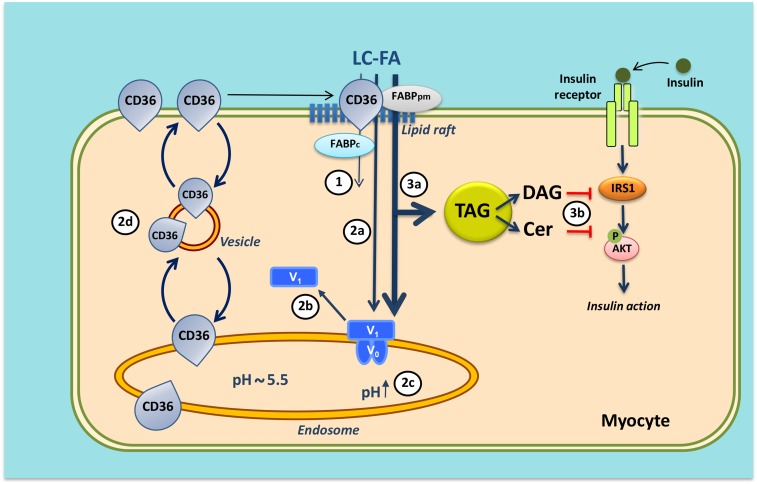
Fig. 4.
Mechanism of lipid-induced v-ATPase inhibition and loss of insulin sensitivity in cardiac myocytes upon lipid over-supply (1). When the supply of long-chain fatty acids (LC-FA) is low, CD36 is primarily found in endosomes. Furthermore, the v-ATPase V0 subcomplex, which is integral to the endosomal membrane, is assembled with the cytosolic V1 subcomplex, allowing for acidification of the endosomal lumen. In this situation, the available LC-FA is metabolized to meet the immediate energy demand of the myocyte. Elevated extracellular LC-FA supply triggers a series of events: increased CD36-mediated LC-FA uptake results in elevated intramyocellular LC-FA levels causing the V1 subcomplex to dissociate (circled 2a); the V1 subcomplex is shifted toward the cytoplasm (circled 2b); the disassembly of v-ATPase leads to endosomal alkalinization (circled 2c); and increased endosomal pH triggers the translocation of CD36 vesicles to the sarcolemma (circled 2d). Upon chronic lipid over-supply, where LC-FA uptake surpasses the metabolic needs, further processes are set in motion: the lipid-induced increase in sarcolemmal CD36 feeds forward to further LC-FA uptake and progressive lipid storage [triacylglycerol (TAG)] (circled 3a); and excess formation of diacylglycerol (DAG) and ceramides (Cer) culminates in impairment of the insulin signaling cascade and, therefore, loss of insulin sensitivity (circled 3b). Modified from (71).