Abstract
Pain is a common problem in children with chronic kidney disease (CKD); however, limited data exist regarding its management. Although most pain is managed pharmacologically, in some instances non-pharmacologic management can aid in safely ameliorating discomfort. Because of the accumulation of toxic metabolites, many common pain medications have adverse effects on kidney function or altered pharmacokinetics in the setting of CKD. Decreased clearance impacts safe dosing of analgesics. The pain management of patients on renal replacement therapy requires an understanding of drug clearance due to the different modalities of dialysis. This educational review highlights pain medications that are safe, albeit often with adjusted dosing, as well as drugs best avoided in the management of pediatric kidney disease. Acetaminophen should be used as a first-line therapy for pain management in children with CKD. Opioids may be added to control moderate to severe pain. Although data are currently lacking, buprenorphine holds promise as a potentially useful drug for the treatment of pain in pediatric patients with CKD. The addition of adjuvant pain medications and non-pharmacologic therapies maybe also be helpful. Despite these options, pain often remains difficult to treat in children with CKD.
Keywords: analgesia, chronic kidney disease, dialysis, nephrology, pain, pediatrics
Introduction
Chronic kidney disease (CKD) refers to irreversible damage to the kidneys. End-stage renal disease (ESRD) is defined as CKD resulting in the need for renal replacement therapy in the form of dialysis or transplantation.1 In 2003, the prevalence of pediatric ESRD in the United States was estimated to be 102 per million, a number that will continue to increase as therapies allow for longer survival.2 CKD alters drug metabolism because of 1) decreased absorption from delayed gastric emptying, increased gastric pH, and bowel wall edema; 2) increased volume of distribution and decreased protein binding; 3) altered metabolism with accumulation of active drug; and 4) decreased elimination due to lower glomerular filtration rate and decreased protein binding, leading to a higher incidence of adverse effects.3
Pain is a subjective experience caused by nociceptors sending signals to the brain meant to help us avoid or decrease the severity of injury. Acute pain is caused by thermal, mechanical, chemical, or inflammatory activation of nociceptive neurons.4 Chronic pain is generally defined as pain lasting longer than 3 months, or longer than the expected healing time for an acute injury.5 This pain can be caused by continued inflammation, damage to neurons, or other mechanisms not fully understood.4,6 Pain intensity can be measured using verbal input (McGills Pain Questionnaire), visual scales (Wong-Baker faces) or numeric scales. The Wong-Baker face pain rating scale is useful in the pediatric population.7
Recently, there has been interest in trying to quantify the prevalence of pain in the pediatric CKD population. Multiple groups have used the National Institutes of Health's Patient-Reported Outcomes Measurement Information System (PROMIS), a validated and publicly accessible question bank, to assess self-reported pain in various diseases. In one study of more than 1000 children with chronic diseases in North Carolina and Texas, almost half of the 384 children with CKD had experienced pain in the past week that interfered with their usual activities.8 This was consistent across the various stages of CKD. These results were echoed by a survey of 233 children with CKD from 14 centers in the United States also using the PROMIS question bank.9 This suggests proper pain management is an important problem in this population and has substantial room for improvement.
Medications recommended for pain management in adults with CKD include acetaminophen (APAP), tramadol, hydromorphone, methadone, fentanyl, gabapentin, and oxycodone.10 Although CKD in adults occurs mostly because of diabetic nephropathy and hypertension, the etiology in children is commonly related to congenital anomalies and glomerular diseases.3 Additionally, in children, development and maturation of the kidneys can modify the pharmacokinetics of drugs and impact drug response. The goal of this article is to discuss the use of analgesic medications in children with kidney disease. Unfortunately, there is a lack of data in children, and the great majority of studies include only adult patients. We extensively reviewed the literature, including the mechanism of action, pharmacokinetics, and safety profile of various medications, to determine the medications and doses that are appropriate to use in children with CKD.
Non-pharmacologic Therapy
Although most pain in the hospital is managed pharmacologically, in some instances non-pharmacologic management can aid in safely ameliorating discomfort. Topical heat or cooling can help with muscle pain, particularly after injury.4 Prospective studies and small randomized trials have suggested that mind-body interventions, such as meditation, can help decrease perception of pain.11 In cases where musculoskeletal pain is a contributing factor, physical therapy can be helpful for decreasing pain and improving quality of life.12,13 Although there may be some benefit, currently there is insufficient evidence supporting the use of acupuncture for pain in patients with CKD.14 In the pediatric population, distraction techniques and involvement of child-life specialists may help with the management of pain.15
Recommended Analgesics
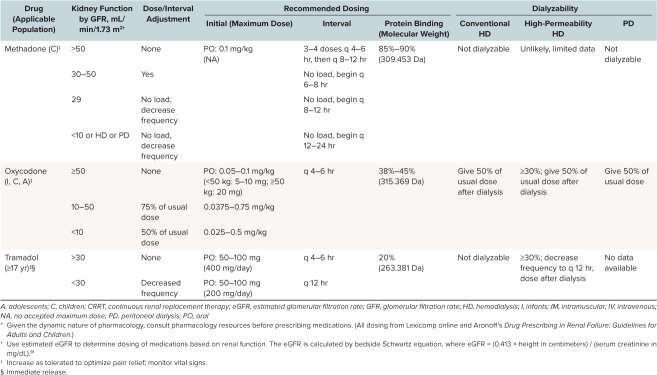
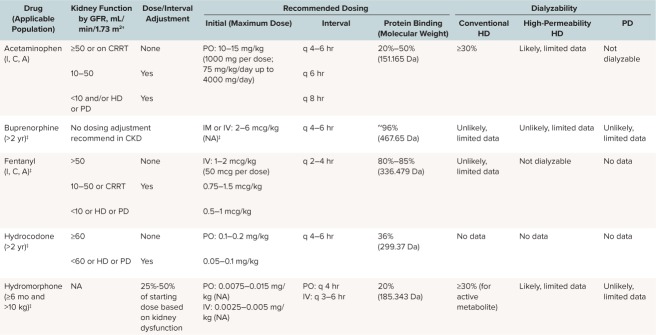
A variety of analgesics are recommended based on severity of pain, conditions associated with the pain, and organ function. Different analgesics may vary in effectiveness depending on the specific characteristics of an individual patient's pain. For example, steroids may help alleviate chronic inflammatory pain, whereas antiepileptic drugs may be effective only for neuropathic pain. Pain control in patients with CKD presents challenges because many analgesics undergo renal metabolism or elimination and thus must be dose adjusted for these patients. The Table summarizes recommended medications as well as renal dosing adjustments.16,17
Table.
Dose Adjustment of Recommended Analgesics in Chronic Kidney Disease (CKD) (cont.) *
Table.
Dose Adjustment of Recommended Analgesics in Chronic Kidney Disease (CKD) *
In 1986, the World Health Organization established a 3-step ladder for pain management which has been widely used in patients with CKD (Figure).10,18 For mild pain, they recommend the use of a non-opiate medication. For patients with moderate pain, lower-potency opioids, including hydromorphone, hydrocodone, or tramadol, may be added to a non-opioid. For severe pain, the addition of higher-potency opioids is recommended. At any step, adjuvants may be prescribed depending on the etiology of the pain.
Figure.
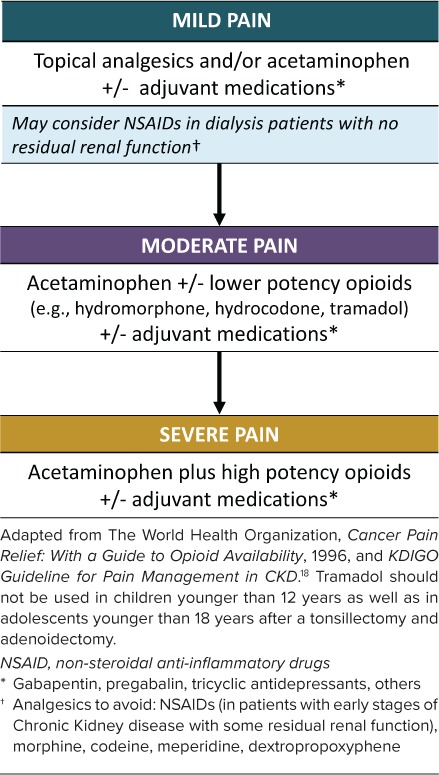
Hierarchy of recommended analgesics for pain control in pediatric chronic kidney disease.
Non-opioids: Acetaminophen. The preferred medication for analgesia in patients with CKD unless otherwise contraindicated is APAP. Although its mechanism is not fully understood, APAP is believed to reduce pain by inhibiting the cyclooxygenase isoenzyme at the peroxidase site, ultimately resulting in a reduction of prostaglandin production.19 It is 20% to 50% protein bound and undergoes hepatic metabolism, making accumulation to toxic concentrations or kidney damage in the setting of CKD less likely.19,20 Some recent case reports suggest that APAP may be dialyzable.21,22
Although some retrospective studies have suggested an association between regular or large doses of APAP and renal toxicity, other cohort studies have found that chronic use of APAP does not correlate with decreased kidney function.23–25 In contrast, overdose of APAP is associated with negative renal consequences. Acute kidney injury can develop after overdose of APAP. Most studies have reported rates to be 2% to 10%26; however, one adult study reported rates of 79% following severe overdose.20
APAP is thought to cause both liver and renal tubular damage via the toxic metabolite N-acetyl-p-benzoquinone-imine (NAPQI), which is produced during APAP metabolism via cytochrome P450 isoenzymes found in both the liver and kidney. When APAP is administered at therapeutic doses, less than 5% of APAP is metabolized to NAPQI. Following overdose, however, depletion of glutathione results in high levels of NAPQI.19 The harmful consequences of overdose should be taken into account when considering large-dose or long-term APAP use. APAP is available in tablet/capsule, chewable tablet, liquid, and injectable forms.
Opioids. Most opioids undergo hepatic biotransformation and are primarily eliminated by renal excretion. Significant renal retention of active or toxic metabolites can occur among advanced CKD patients and lead to central nervous system and respiratory depression as well as hypotension.4
Hydromorphone. Hydromorphone is a synthetic morphine derivative that is significantly more potent than morphine. It may be used as a second-line agent for control of moderate pain. Hydromorphone is hepatically metabolized to hydromorphone-3-glucuronide, dihydromorphine, and dihydroisomorphine as well as miniscule amounts of other metabolites. It is 20% protein bound.17 All metabolites are renally excreted and therefore accumulate in CKD. The area under the curve for plasma concentration of hydromorphone has been found to be 2-fold higher in patients with moderate renal failure (estimated GFR, 40–60 mL/min/1.73 m2), and 4-fold higher in those with severe renal impairment (estimated GFR, <30 mL/min/1.73 m2), compared with those with normal renal function.27–29 Thus, lower starting doses with or without increased dosing intervals may be needed depending on the degree of renal impairment.29–31 Hydromorphone is 40% to 55% cleared by hemodialysis.32 Case reports and small studies have demonstrated safety and efficacy in adults with ESRD. Although hydromorphone-3-glucuronide accumulates in these patients, it has not been documented to cause adverse effects.30,33,34 No data currently exist on the safety and efficacy of hydromorphone in pediatric CKD patients. Hydromorphone is available in tablet, suppository, liquid, and injectable forms.
Hydrocodone. Hydrocodone is a semisynthetic opioid derived from codeine.35 It can be considered as a second-line medication in the management of moderate pain in patients with CKD.4 It is 36% protein bound. Hydrocodone is hepatically metabolized by CYP450 2D6 to hydromorphone, which has a higher affinity for the mu opioid receptor compared with hydrocodone. It undergoes N-demethylation via CYP3A4 to its major metabolite, norhydrocodone; and remaining metabolism occurs via other non-CYP pathways.36 It is excreted via urine. The concomitant use of hydrocodone with cytochrome P450 3A4 inhibitors may result in an increase in plasma concentrations of hydrocodone, which could increase adverse drug effects, including respiratory depression. No data are available on the clearance of hydrocodone by patients who are undergoing dialysis. In the United States, hydrocodone is currently only available as a tablet, solution, or elixir. The hydrocodone-only extended-release tablets should not be used in pediatric patients with CKD.
Fentanyl. Fentanyl is a potent synthetic opioid with a short half-life. It is primarily metabolized by the liver to inactive metabolites (>99%). In healthy patients, it is mostly excreted in the urine, with a small amount excreted in the feces.37 Because of the high potency of fentanyl, it should be used with caution in opiate-naive patients.38 Case reports and molecular analysis of fentanyl indicate that it is highly protein (80%–85%) and lipid bound and unlikely to be cleared by dialysis.17 Rather than traditional clearance by diffusion or convection, it is hypothesized that the small amount of fentanyl removed during dialysis is absorbed into the membrane itself.39–41 Data on the safety of fentanyl in patients with renal dysfunction are conflicting. A small case series of perioperative fentanyl use in patients with ESRD suggested that fentanyl may be safe and effective.41 However, a randomized, controlled trial of 8 adult patients suggested that patients with the highest blood urea nitrogen levels had the slowest fentanyl clearance, and that this could be clinically correlated with the need for postoperative respiratory support.42 Despite this possibility, fentanyl is commonly used for pain management in patients with CKD because of its effectiveness as an analgesic and minimal dose adjustments needed for patients with renal impairment. Long-term use of fentanyl is associated with tachyphylaxis and hyperalgesia. Hyperalgesia should be considered in patients with uncontrolled pain despite escalating fentanyl doses. Fentanyl is available in transdermal patch, nasal spray, sublingual tablet, lozenge, and injectable forms. Currently, there are no recommendations about the safety of transdermal fentanyl systems or other formulations of fentanyl aside from intravenous in CKD.
Methadone. Methadone is a synthetic, long-acting mu opioid agonist. It acts as a mu opioid receptor agonist as well as an antagonist at the N-methyl-D-aspartate receptor, and it has been used in refractory neuropathic pain in many patients.43,44 It has a higher bioavailability and a significantly longer half-life than either morphine or hydromorphone.45 It is 85% to 90% protein bound and is metabolized in the liver to inactive metabolites, which are excreted via both the urine and feces.17 It has been demonstrated that anuric patients are able to effectively excrete methadone via the gut; however; constipation, a common side effect of opioids, could theoretically complicate this process. Methadone has been studied in only small numbers of ESRD patients but is generally considered safe in adults with CKD with careful titration while monitoring vital signs.32,46 Of note, methadone is not well removed by hemodialysis. Other safety parameters to consider include the potential for many drug interactions secondary to its metabolism by cytochrome P450, as well as QTc prolongation which may require close monitoring.47 Methadone is available in tablet, dispersible tablet, liquid, and injectable forms.
Topical Analgesics
For musculoskeletal pain, analgesics in the form of creams or sprays that can be applied topically with limited systemic absorption may allow for pain relief.48 Non-steroidal anti-inflammatory drugs (NSAIDs), including diclofenac, ibuprofen, and ketoprofen, are the most widely studied topical analgesics, and they have demonstrated short-term efficacy greater than placebo.49 Topical NSAIDs may result in lower blood levels and reduced systemic side effects compared with oral administration.6 Despite the lower serum drug levels associated with topical therapy, insufficient data exist regarding the safety of topical NSAIDs in any patients with CKD, including children. Topical lidocaine, capsaicin, and amitriptyline have limited evidence of therapeutic effect.50
Medications With Insufficient Evidence
Buprenorphine. Buprenorphine is an effective, long-acting opioid derivative that acts as a mixed-partial agonist at mu opioid receptors. It is metabolized in the liver to norbuprenorphine, buprenorphine-3-glucuronide, and norbuprenorphine-3-glucuronide, which are excreted mostly via bile and feces, and to a lesser extent via urine.51,52 Although the metabolites have lower receptor affinity than the mother compound, the metabolites have been shown to have an antinociceptive effect and cause some sedation, albeit while having no effect on respiratory rate, in mice.53 In humans, it has been shown to have a “ceiling” effect, with larger doses above a certain threshold plateauing in clinical effect. In healthy, opiate-naive adults challenged with increasing doses of buprenorphine, respiratory rate reached a floor level of approximately 12 breaths per minute regardless of dose.54 Single-dose buprenorphine has been shown to have similar pharmacokinetics in renally impaired and healthy patients. In patients with CKD given continuous infusion, there was some increase in concentration of metabolites.55 However, in a 1-week-long study of 10 adults with ESRD on dialysis, plasma levels of metabolites did not accumulate, and no dose adjustment or adverse side effects were noted.52 Buprenorphine and its metabolites are not cleared by hemodialysis, allowing for steady plasma levels and pain relief across dialysis sessions.52 This suggests a favorable safety profile with lower risk of life-threatening reactions with overdose for buprenorphine compared with other opiates. Although data in children are lacking, the favorable safety profile in adults suggests that buprenorphine may hold promise as a useful drug for the treatment of pain in pediatric patients with CKD. If needed, a consultation with a pain specialist is recommended prior to using this medication in pediatric patients. Currently, buprenorphine is only available in injectable form and as a sublingual tablet, which makes chronic use in small children challenging.
Oxycodone. Oxycodone is metabolized in the liver and yields multiple metabolites, one of which is active.56 It is 38% to 45% protein bound.17 Although only approximately 10% is excreted unchanged in the urine, the half-life is unpredictably prolonged in patients with CKD.57 There are case reports of patients on oxycodone experiencing suppression of the central nervous system with usual doses in the presence of renal failure.58 Although the safety of oxycodone in healthy children has been established, more research is necessary before the safety of oxycodone in pediatric CKD can be confidently stated.10,59 Oxycodone is available in tablet and liquid forms.
Tramadol. Tramadol is a synthetic opioid derivative that is metabolized by the liver and excreted by the kidneys. Tramadol acts via multiple pathways to decrease pain, and although it is considered a similarly strong analgesic as morphine, it has a comparatively less potent effect at mu opioid receptors. Tramadol is 20% protein bound.17 The lack of effect at mu receptors and the low degree of protein binding decrease the risk of side effects, such as respiratory depression and hemodynamic instability, at large doses, and also decrease (but do not eliminate) the potential for addiction.60 Tramodol is a prodrug that is converted to active metabolites by cytochrome P450 enzymes. Because tramadol is renally excreted, the half-life may increase up to 2-fold in patients with severe kidney disease. This prolonged half-life can increase the risk of seizures and respiratory depression. Tramadol can also cause serotonin syndrome if taken in combination with other drugs that increase serotonin concentrations.34 It is partially removed by hemodialysis. There is very limited literature evaluating the use of tramadol in patients with CKD. Tramadol is not approved by the US Food and Drug Administration (FDA) for use in children because it can lead to fatal respiratory depression. In September 2015, the FDA published a drug safety communication warning that certain genetically predisposed children could rapidly metabolize tramadol, leading to an accumulation of sedating metabolites and potentially causing death.61 As per the FDA recommendations issued in 2015, tramadol is now contraindicated for pain management in all children younger than 12 years as well as in adolescents younger than 18 years after a tonsillectomy and adenoidectomy.61 Tramadol is available as a tablet or suspension.
Analgesics to Avoid
NSAIDs. NSAIDs are commonly used non-opioid analgesics. They block prostaglandin synthesis, which decreases perception of pain. However, the blockade of prostaglandins causes adverse effects on the kidneys by impairing autoregulation of renal blood flow, and thus decreases glomerular filtration rate.38,62 In healthy adults, they can worsen hypertension, which is often a problem in patients with CKD.63 Given these concerns, NSAIDs are generally considered to be contraindicated in children with CKD. However, if needed they may be an option for patients without any residual renal function. Additionally, NSAIDs should be avoided in patients exposed to radiocontrast or those on other nephrotoxic medications, including diuretics, angiotensin-converting enzyme inhibitors, and angiotensin receptor blockers.64 If NSAIDs must be used, short-acting agents (e.g., ibuprofen, ketoprofen) are preferred. Aspirin has analgesic and anti-inflammatory effects only at intermediate and large doses. At larger doses, the chronic use of aspirin leads to renal vasoconstriction, interstitial nephritis, and decline in renal function.65,66 Further, the use of aspirin has declined because of concern for association with Reye syndrome in children younger than 12 years.67 Sulindac and salsalate are weaker inhibitors of prostaglandin synthesis and may have a renal-sparing effect.6 However, these have not been studied in the pediatric population.
Morphine. Morphine is a well-studied opiate not recommended for use in CKD.34 It is metabolized in the liver to morphine-3-glucuronide and morphine-6-glucuronide. Both of these metabolites are excreted via the urine and can accumulate in patients with CKD.68 Morphine-6-glucuronide is an active metabolite, so its accumulation in patients with CKD can have dangerous sedating effects.69 It crosses the blood-brain barrier slowly, and its effects are not quickly reversed by hemodialysis.59
Codeine. Codeine is a prodrug that is converted to active metabolites by cytochrome P450 enzymes. It is an opioid that metabolizes to codeine glucuronide, morphine, and morphine glucuronide. Its clearance is decreased in patients with impaired kidney function and, because it is metabolized to morphine, it is subject to the same risks of use in the setting of renal impairment.70 Furthermore, there have been serious adverse events, including death, in children who have a polymorphism in cytochrome P450, causing them to be ultrarapid metabolizers. For this reason, codeine is rarely used in any pediatric population. Recently the FDA released a warning statement against the use of codeine to treat pain or cough in children younger than 12 years.71
Meperidine. Meperidine is a synthetic opioid which is hepatically metabolized and renally excreted. Normeperidine, its major metabolite, accumulates in CKD and causes central nervous system excitability, predisposing patients to seizures. This effect is not reversible by naloxone. Meperidine is a drug best avoided in patients with CKD.72,73 As of 2007 and 2016, the Institute for Safe Medication Practices and the American Pain Society, respectively, do not recommend meperidine as an analgesic.16
Dextropropoxyphene. Dextropropoxyphene is a weak opioid that is no longer available in the United States. It was briefly available as a combination analgesic with APAP, but its many side effects, drug-drug interactions, weak analgesic properties, and potential for abuse resulted in its removal from the US and European Union markets. It is still available in some parts of the world. Its main metabolite undergoes urinary excretion, and buildup of both the drug and its metabolite in CKD can cause fatal arrhythmias, respiratory toxicity, and adverse neurologic symptoms.74,75 Because of these concerns, dextropropoxyphene is contraindicated in patients with ESRD.
Adjuvant Medications
Benzodiazepines. Benzodiazepines are a class of medication used for sedation and anxiolysis. Benzodiazepines, such as midazolam, diazepam, and lorazepam, are commonly used in children for non-analgesic purposes, such as procedural sedation or seizure control. However, they can also be used as an ancillary to pain management in some circumstances. Benzodiazepines have an indirect effect in pain control related to their psychotropic properties.76 For example, in adults, benzodiazepines are commonly prescribed to dialysis patients for the treatment of anxiety, restless leg syndrome, and/or insomnia. Studies from 1995–2002 have reported that 10% to 25% of adult dialysis patients take benzodiazepines. For comparison, use in the general population reportedly ranges from 2% to 6%.21 Benzodiazepines work via stimulation of inhibitory γ-aminobutyric acid receptors. They are highly protein bound, hepatically metabolized, and renally eliminated.11,12 They are only partially cleared by dialysis (approximately 50%).22 According to a study analyzing the US Renal Data System from 1996–1997, the most commonly prescribed benzodiazepines were temazepam (not approved for use in children) and lorazepam.23
Benzodiazepines are classified in terms of their elimination half-life: 1) short-acting, for example, midazolam (median elimination half-life of 1–12 hours); 2) intermediate-acting, for example, lorazepam and alprazolam (median elimination half-life of 12–40 hours); and 3) long-acting, for example, diazepam (median elimination half-life of 40–250 hours). Longer-acting benzodiazepines form active metabolites. Midazolam, one of the short-acting benzodiazepines, produces no active metabolites. However, diazepam produces the active metabolites oxazepam, desmethyldiazepam, and temazepam, which further increases the duration of drug action and should be a serious consideration in patients with CKD. In general, benzodiazepines should be used with caution in patients with renal dysfunction, especially if used concomitantly with other medications that cause respiratory depression.11
Neuropathic Pain
Gabapentin. Gabapentin can be a useful non-opioid analgesic for patients with neuropathic, as opposed to inflammatory or musculoskeletal, pain.77 Gabapentin helps with pain by modulating the release of excitatory neurotransmitters.11 It is predominantly excreted in the urine, so the dose must be reduced in patients with kidney disease. It is 3% protein bound and does not have significant drug-drug interactions.12,24 Approximately 35% is cleared by hemodialysis; clearance by peritoneal dialysis is unknown.12,25,26 Some genetic variability in metabolism exists. Organic cation transporter 1 (OCTN1) contributes to active tubular secretion of gabapentin, and this effect may be diminished or absent in individuals carrying the OCTN1-L503F polymorphism.27 Although gabapentin can accumulate in patients on dialysis and cause adverse effects, such as decreased level of consciousness, ataxia, dizziness, myoclonus, and confusion, side effects are uncommon.24 Although gabapentin has not to our knowledge been studied in pediatric CKD, it seems reasonable to extrapolate that it would be safe based on the adult CKD data and the knowledge that gabapentin is commonly used in other pediatric populations.
Pregabalin. Pregabalin is an antiepileptic drug approved for use in neuropathic pain.11 It is minimally protein bound and mainly renally excreted in unmetabolized form. Clearance is proportional to creatinine clearance, and dosages must be reduced in patients with CKD.28 In adults, it can be a useful non-opioid adjunct for management of neuropathic pain as well as other symptoms, such as restless leg syndrome and pruritis, associated with CKD, if the dose is adjusted. However, it is not approved for pediatric use and not commonly used in this population.
Tricyclic Antidepressants. Tricyclic antidepressants (TCAs) are a class of medication that, like gabapentin, can be used for neuropathic pain. They reduce pain via providing sodium channel blockade and reducing norepinephrine and serotonin reuptake. With their anticholinergic properties, they commonly cause adverse side effects, such as orthostatic hypotension, urinary retention, sedation, confusion, dry mouth, constipation, weight gain, and cardiac conduction block.29 TCAs are classified as either 1) tertiary amines, for example, amitriptyline, imipramine, doxepin, trimipramine, and clomipramine; or, 2) secondary amines, including desipramine, nortriptyline, and protriotyline.78 The tertiary amines generally cause more side effects. Both desipramine and amitriptyline have been used for the management of neuropathic pain in CKD, with desipramine resulting in less severe anticholinergic side effects than amitriptyline.26 TCAs are highly protein bound.11 They are hepatically metabolized by cytochrome P450, creating a large potential for drug-drug interactions.29,30 TCAs are renally excreted and have a markedly increased half-life in patients with ESRD. They are not substantially cleared by dialysis.30 Given these limitations, they are not considered first-line medications for neuropathic pain in children or adults but can be considered if other agents are unavailable or are unsuccessful.
Other Anticonvulsants. Carbamazepine and oxcarbazepine stabilize voltage-gated sodium channels and may be used as an adjuvant in neuropathic pain. Carbamazepine is hepatically eliminated and does not require renal dosing. Oxcarbazepine is hepatically metabolized but renally eliminated. Dose adjustment is recommended because its active metabolite can build up in patients with reduced renal function.79
Conclusions
Pain is a significant problem in pediatric patients with CKD. Limited data exist regarding pain management in these children. Most current recommendations are based on adult studies. We consider APAP safe as a first-line analgesic for pediatric CKD, whereas morphine, codeine, meperidine, and NSAIDs are best avoided. We would consider hydromorphone, hydrocodone, and tramadol as medications for patients with moderate pain. Fentanyl is a reasonable opiate to consider in severe pain because it is nearly entirely metabolized by the liver, and thus requires minimal dose adjustments in patients with CKD. Its potency also makes it an effective choice, although caution should be exercised, particularly in children, because of the potentially lethal effects of overdose. TCAs have some evidence of safety but are less than ideal because of their extensive side effect profile. Buprenorphine appears to be a promising drug for pain management in CKD, although further research in pediatric populations is necessary before use in pediatric CKD patients can be recommended.
We extensively reviewed the literature to determine the medications and doses that are appropriate to use in children with CKD. However, children are not young adults. When prescribing medications, we recommend using a reliable pediatric reference source. Adjusting dose of medications for age, size, and clinical context is essential to administering drugs safely and effectively.80
ABBREVIATIONS
- APAP
acetaminophen
- CKD
chronic kidney disease
- ESRD
end-stage renal disease
- FDA
US Food and Drug Administration
- NAPQI
N-acetyl-p-benzoquinone-imine
- NSAIDs
non-steroidal anti-inflammatory drugs
- OCTN1
Organic cation transporter 1
- PROMIS
Patient-Reported Outcomes Measurement Information System
- TCAs
tricyclic antidepressants
Footnotes
Disclosure The authors declare no conflicts or financial interest in any product or service mentioned in the manuscript, including grants, equipment, medications, employment, gifts, and honoraria.
Copyright Published by the Pediatric Pharmacy Advocacy Group. All rights reserved. For permissions, email: matthew.helms@ppag.org
REFERENCES
- 1. National Kidney Foundation. . KDOQI clinical practice guidelines for chronic kidney disease: evaluation, classification and stratification. Am J Kidney Dis. 2002; 39 2 suppl 1: S1– S266. [PubMed] [Google Scholar]
- 2. National Institute of Diabetes and Digestive and Kidney Diseases. . USRDS 2012 Annual Data Report: Atlas of Chronic Kidney Disease and End-Stage Renal Disease in the United States. Bethesda, MD: National Institutes of Health; 2012. https://www.usrds.org/2012/pdf/v1_00intro_12.pdf. Accessed May 27, 2017. [Google Scholar]
- 3. Rodieux F, Wilbaux M, van den Anker JN, . et al. Effect of kidney function on drug kinetics and dosing in neonates, infants, and children. Clin Pharmacokinet. 2015; 54 12: 1183– 1204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Pham PCT, Toscano E, Pham PMT, . et al. Pain management in patients with chronic kidney disease. NDT Plus. 2009; 2 2: 111– 118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Task Force on Taxonomy of the International Association for the Study of Pain. . Classification of Chronic Pain: Descriptions of Chronic Pain Syndromes and Definitions of Pain Terms. 2nd ed Seattle, WA: IASP Press; 1994. [Google Scholar]
- 6. Pham PC, Khaing K, Sievers TM, . et al. 2017 update on pain management in patients with chronic kidney disease. Clin Kidney J. 2017; 10 5: 688– 697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Wong DL, Baker CM.. Pain in children: comparison of assessment scales. Pediatr Nurs. 1988; 14 1: 9– 17. [PubMed] [Google Scholar]
- 8. DeWalt DA, Gross HE, Gipson DS, . et al. PROMIS® pediatric self-report scales distinguish subgroups of children within and across six common pediatric chronic health conditions. Qual Life Res. 2015; 24 9: 2195– 2208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Selewski DT, Massengill SF, Troost JP, . et al. Gaining the Patient Reported Outcomes Measurement Information System (PROMIS) perspective in chronic kidney disease: a Midwest Pediatric Nephrology Consortium study. Pediatr Nephrol Berl Ger. 2014; 29 12: 2347– 2356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Davison S, Koncicki H, Brennan F.. Chronic pain in CKD: a scoping review. Semin Dial. 2014; 27 2: 188– 204. [DOI] [PubMed] [Google Scholar]
- 11. Rosenzweig S, Greeson JM, Reibel DK, . et al. Mindfulness-based stress reduction for chronic pain conditions: variation in treatment outcomes and role of home meditation practice. J Psychosom Res. 2010; 68 1: 29– 36. [DOI] [PubMed] [Google Scholar]
- 12. Crossley K, Bennell K, Green S, . et al. Physical therapy for patellofemoral pain a randomized, double-blinded, placebo-controlled trial. Am J Sports Med. 2002; 30 6: 857– 865. [DOI] [PubMed] [Google Scholar]
- 13. Feine JS, Lund JP.. An assessment of the efficacy of physical therapy and physical modalities for the control of chronic musculoskeletal pain. Pain. 1997; 71 1: 5– 23. [DOI] [PubMed] [Google Scholar]
- 14. Kim KH, Lee MS, Kim TH, . et al. Acupuncture and related interventions for symptoms of chronic kidney disease. Cochrane Database Syst Rev. 2016; 6: CD009440. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Bandstra NF, Skinner L, Leblanc C, . et al. The role of child life in pediatric pain management: a survey of child life specialists. J Pain Off J Am Pain Soc. 2008; 9 4: 320– 329. [DOI] [PubMed] [Google Scholar]
- 16. Lexicomp Online, Pediatric & Neonatal Lexi-Drugs, Hudson, Ohio: Lexi-Comp, Inc. Pages on acetaminophen, buprenorphine, fentanyl, hydrocodone, hydromorphone, methadone, oxycodone, and tramadol. Accessed 9/2/2016-12/6/2017. [Google Scholar]
- 17. Drug prescribing in renal failure: dosing guidelines for adults and children. Eds: Aronoff GR, Bennett WM.. American College of Physicians. 2007: 17– 20, 135– 137. [DOI] [PubMed] [Google Scholar]
- 18. World Health Organization. . Cancer pain relief: With a guide to opioid availability. 2nd ed Geneva, Switzerland: World Health Organization; 1996: 3– 36. [Google Scholar]
- 19. Mazer M, Perrone J.. Acetaminophen-induced nephrotoxicity: pathophysiology, clinical manifestations, and management. J Med Toxicol. 2008; 4 1: 2– 6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. O'Riordan A, Brummell Z, Sizer E, . et al. Acute kidney injury in patients admitted to a liver intensive therapy unit with paracetamol-induced hepatotoxicity. Nephrol Dial Transplant. 2011; 26 11: 3501– 3508. [DOI] [PubMed] [Google Scholar]
- 21. Serjeant L, Evans J, Sampaziotis F, . et al. Haemodialysis in acute paracetamol poisoning. BMJ Case Rep. 2017; 2017: pii: bcr2016218667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Ghannoum M, Kazim S, Grunbaum AM, . et al. Massive acetaminophen overdose: effect of hemodialysis on acetaminophen and acetylcysteine kinetics. Clin Toxicol (Phila). 2016; 54 6: 519– 522. [DOI] [PubMed] [Google Scholar]
- 23. Barrett BJ. Acetaminophen and adverse chronic renal outcomes: an appraisal of the epidemiologic evidence. Am J Kidney Dis. 1996; 28 1: S14– S19. [DOI] [PubMed] [Google Scholar]
- 24. Kurth T, Glynn RJ, Walker AM, . et al. Analgesic use and change in kidney function in apparently healthy men. Am J Kidney Dis. 2003; 42 2: 234– 244. [DOI] [PubMed] [Google Scholar]
- 25. Perneger TV, Whelton PK, Klag MJ.. Risk of kidney failure associated with the use of acetaminophen, aspirin, and nonsteroidal antiinflammatory drugs. N Engl J Med. 1994; 331 25: 1675– 1679. [DOI] [PubMed] [Google Scholar]
- 26. Stollings JL, Wheeler AP, Rice TW.. Incidence and characterization of acute kidney injury after acetaminophen overdose. J Crit Care. 2016; 35: 191– 194. [DOI] [PubMed] [Google Scholar]
- 27. Zernikow B, Michel E, Craig F, . et al. Pediatric palliative care: use of opioids for the management of pain. Paediatr Drugs. 2009; 11 2: 129– 151. [DOI] [PubMed] [Google Scholar]
- 28. Zheng M, McErlane KM, Ong MC.. Hydromorphone metabolites: isolation and identification from pooled urine samples of a cancer patient. Xenobiotica. 2002; 32 5: 427– 439. [DOI] [PubMed] [Google Scholar]
- 29. Durnin C, Hind ID, Wickens MM, . et al. Pharmacokinetics of oral immediate-release hydromorphone (Dilaudid IR) in subjects with renal impairment. Proc West Pharmacol Soc. 2001; 44: 81– 82. [PubMed] [Google Scholar]
- 30. Babul N, Darke AC, Hagen N.. Hydromorphone metabolite accumulation in renal failure. J Pain Symptom Manage. 1995; 10 3: 184– 186. [DOI] [PubMed] [Google Scholar]
- 31. Lee MA, Leng MEF, Tiernan EJJ.. Retrospective study of the use of hydromorphone in palliative care patients with normal and abnormal urea and creatinine. Palliat Med. 2001; 15 1: 26– 34. [DOI] [PubMed] [Google Scholar]
- 32. Perlman R, Giladi H, Brecht K, . et al. Intradialytic clearance of opioids: methadone versus hydromorphone. Pain. 2013; 154 12: 2794– 2800. [DOI] [PubMed] [Google Scholar]
- 33. Davison SN, Mayo PR.. Pain management in chronic kidney disease: the pharmacokinetics and pharmacodynamics of hydromorphone and hydromorphone-3-glucuronide in hemodialysis patients. J Opioid Manag. 2008; 4 6: 335– 336, 339– 344. [PubMed] [Google Scholar]
- 34. Parmar MS, Parmar KS.. Management of acute and postoperative pain in chronic kidney disease. F1000Res. 2013; 2: 28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Singla A, Sloan P.. Pharmacokinetic evaluation of hydrocodone/acetaminophen for pain management. J Opioid Manag. 2013; 9 1: 71– 80. [DOI] [PubMed] [Google Scholar]
- 36. Hutchinson MR, Menelaou A, Foster DJR, . et al. CYP2D6 and CYP3A4 involvement in the primary oxidative metabolism of hydrocodone by human liver microsomes. Br J Clin Pharmacol. 2004; 57 3: 287– 297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. McClain DA, Hug CC.. Intravenous fentanyl kinetics. Clin Pharmacol Ther. 1980; 28 1: 106– 114. [DOI] [PubMed] [Google Scholar]
- 38. Kurella M, Bennett WM, Chertow GM.. Analgesia in patients with ESRD: a review of available evidence. Am J Kidney Dis. 2003; 42 2: 217– 228. [DOI] [PubMed] [Google Scholar]
- 39. Bastani B, Jamal JA.. Removal of morphine but not fentanyl during haemodialysis. Nephrol Dial Transplant. 1997; 12 12: 2802– 2804. [DOI] [PubMed] [Google Scholar]
- 40. Joh J, Sila MK, Bastani B.. Nondialyzability of fentanyl with high-efficiency and high-flux membranes: Anesth Analg. 1998; 86 2: 447. [DOI] [PubMed] [Google Scholar]
- 41. Karanikolas M, Aretha D, Kiekkas P, . et al. Intravenous fentanyl patient-controlled analgesia for perioperative treatment of neuropathic/ischaemic pain in haemodialysis patients: a case series. J Clin Pharm Ther. 2010; 35 5: 603– 608. [DOI] [PubMed] [Google Scholar]
- 42. Koehntop DE, Rodman JH.. Fentanyl pharmacokinetics in patients undergoing renal transplantation. Pharmacotherapy. 1997; 17 4: 746– 752. [PubMed] [Google Scholar]
- 43. Mizoguchi H, Watanabe C, Yonezawa A, . et al. New therapy for neuropathic pain. Int Rev Neurobiol. 2009; 85: 249– 260. [DOI] [PubMed] [Google Scholar]
- 44. Moulin DE, Palma D, Watling C, . et al. Methadone in the management of intractable neuropathic noncancer pain. Can J Neurol Sci. 2005; 32 3: 340– 343. [DOI] [PubMed] [Google Scholar]
- 45. Lugo RA, Satterfield KL, Kern SE.. Pharmacokinetics of methadone. J Pain Palliat Care Pharmacother. 2005; 19 4: 13– 24. [PubMed] [Google Scholar]
- 46. Kreek MJ, Schecter AJ, Gutjahr CL, . et al. Methadone use in patients with chronic renal disease. Drug Alcohol Depend. 1980; 5 3: 197– 205. [DOI] [PubMed] [Google Scholar]
- 47. Atkinson TJ, Fudin J, Wegrzyn EL, . et al. Dialysis, opioids, and pain management: where's the evidence? Pract Pain Manag. 2014; 14 8: 49– 57. [Google Scholar]
- 48. Argoff CE. Topical analgesics in the management of acute and chronic pain. Mayo Clin Proc. 2013; 88 2: 195– 205. [DOI] [PubMed] [Google Scholar]
- 49. Mason L, Moore RA, Edwards JE, . et al. Topical NSAIDs for chronic musculoskeletal pain: systematic review and meta-analysis. BMC Musculoskelet Disord. 2004; 5: 28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Attal N, Bouhassira D.. Pharmacotherapy of neuropathic pain: which drugs, which treatment algorithms? Pain. 2015; 156 suppl 1: S104– S114. [DOI] [PubMed] [Google Scholar]
- 51. Cone EJ, Gorodetzky CW, Yousefnejad D, . et al. The metabolism and excretion of buprenorphine in humans. Drug Metab Dispos Biol Fate Chem. 1984; 12 5: 577– 581. [PubMed] [Google Scholar]
- 52. Filitz J, Griessinger N, Sittl R, . et al. Effects of intermittent hemodialysis on buprenorphine and norbuprenorphine plasma concentrations in chronic pain patients treated with transdermal buprenorphine. Eur J Pain. 2006; 10 8: 743– 743. [DOI] [PubMed] [Google Scholar]
- 53. Brown SM, Holtzman M, Kim T, . et al. Buprenorphine metabolites, buprenorphine-3-glucuronide and norbuprenorphine-3-glucuronide, are biologically active. Anesthesiology. 2011; 115 6: 1251– 1260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Walsh SL, Preston KL, Stitzer ML, . et al. Clinical pharmacology of buprenorphine: ceiling effects at high doses. Clin Pharmacol Ther. 1994; 55 5: 569– 580. [DOI] [PubMed] [Google Scholar]
- 55. Hand CW, Sear JW, Uppington J, . et al. Buprenorphine disposition in patients with renal impairment: single and continuous dosing, with special reference to metabolites. Br J Anaesth. 1990; 64 3: 276– 282. [DOI] [PubMed] [Google Scholar]
- 56. Kaiko RF, Benziger DP, Fitzmartin RD, . et al. Pharmacokinetic-pharmacodynamic relationships of controlled-release oxycodone. Clin Pharmacol Ther. 1996; 59 1: 52– 61. [DOI] [PubMed] [Google Scholar]
- 57. Kirvela M, Lindgren L, Seppala T, . et al. The pharmacokinetics of oxycodone in uremic patients undergoing renal transplantation. J Clin Anesth. 1996; 8 1: 13– 18. [DOI] [PubMed] [Google Scholar]
- 58. Fitzgerald J. Narcotic analgesics in renal failure. Conn Med. 1991; 55 12: 701– 704. [PubMed] [Google Scholar]
- 59. Dean M. Opioids in renal failure and dialysis patients. J Pain Symptom Manage. 2004; 28 5: 497– 504. [DOI] [PubMed] [Google Scholar]
- 60. Grond S, Sablotzki A.. Clinical pharmacology of tramadol. Clin Pharmacokinet. 2012; 43 13: 879– 923. [DOI] [PubMed] [Google Scholar]
- 61. US Food and Drug Administration. . FDA drug safety communication: FDA evaluating the risks of using the pain medicine tramadol in children aged 17 and younger. September 2015. https://www.fda.gov/Drugs/DrugSafety/ucm549679.htm. Accessed April 19, 2018.
- 62. Tan SY, Shapiro R, Kish MA.. Reversible acute renal failure induced by indomethacin. JAMA. 1979; 241 25: 2732– 2733. [PubMed] [Google Scholar]
- 63. Pope JE, Anderson JJ, Felson DT.. A meta-analysis of the effects of nonsteroidal anti-inflammatory drugs on blood pressure. Arch Intern Med. 1993; 153 4: 477– 484. [PubMed] [Google Scholar]
- 64. Lapi F, Azoulay L, Yin H, . et al. Concurrent use of diuretics, angiotensin converting enzyme inhibitors, and angiotensin receptor blockers with non-steroidal anti-inflammatory drugs and risk of acute kidney injury: nested case-control study. BMJ. 2013; 346: e8525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65. Ibáñez L, Morlans M, Vidal X, . et al. Case-control study of regular analgesic and nonsteroidal anti-inflammatory use and end-stage renal disease. Kidney Int. 2005; 67 6: 2393– 2398. [DOI] [PubMed] [Google Scholar]
- 66. Fored CM, Ejerblad E, Lindblad P, . et al. Acetaminophen, aspirin, and chronic renal failure. N Engl J Med. 2001; 345 25: 1801– 1808. [DOI] [PubMed] [Google Scholar]
- 67. Mann RD. Reye's syndrome and aspirin. J R Coll Gen Pract. 1986; 36 290: 418– 421. [PMC free article] [PubMed] [Google Scholar]
- 68. Peterson GM, Randall CT, Paterson J.. Plasma levels of morphine and morphine glucuronides in the treatment of cancer pain: relationship to renal function and route of administration. Eur J Clin Pharmacol. 1990; 38 2: 121– 124. [DOI] [PubMed] [Google Scholar]
- 69. Böger RH. Renal impairment: a challenge for opioid treatment?: the role of buprenorphine. Palliat Med. 2006; 20 8 suppl: 17– 23. [PubMed] [Google Scholar]
- 70. Guay DR, Awni WM, Findlay JW, . et al. Pharmacokinetics and pharmacodynamics of codeine in end-stage renal disease. Clin Pharmacol Ther. 1988; 43 1: 63– 71. [DOI] [PubMed] [Google Scholar]
- 71. US Food and Drug Administration. . Drug safety communication: FDA restricts use of prescription codeine pain and cough medicines and tramadol pain medicines in children; recommends against use in breastfeeding women. 2017. https://www.fda.gov/Drugs/DrugSafety/ucm549679.htm. Accessed April 19, 2018.
- 72. Armstrong PJ, Bersten A.. Normeperidine toxicity. Anesth Analg. 1986; 65 5: 536– 538. [PubMed] [Google Scholar]
- 73. Clark RF, Wei EM, Anderson PO.. Meperidine: therapeutic use and toxicity. J Emerg Med. 1995; 13 6: 797– 802. [DOI] [PubMed] [Google Scholar]
- 74. Barkin RL, Barkin SJ, Barkin DS.. Propoxyphene (dextropropoxyphene): a critical review of a weak opioid analgesic that should remain in antiquity. Am J Ther. 2006; 13 6: 534– 542. [DOI] [PubMed] [Google Scholar]
- 75. Gibson TP, Giacomini KM, Briggs WA, . et al. Propoxyphene and norpropoxyphene plasma concentrations in the anephric patient. Clin Pharmacol Ther. 1980; 27 5: 665– 670. [DOI] [PubMed] [Google Scholar]
- 76. Reddy S, Patt RB.. The benzodiazepines as adjuvant analgesics. J Pain Symptom Manage. 1994; 9 8: 510– 514. [DOI] [PubMed] [Google Scholar]
- 77. Wiffen PJ, Derry S, Bell RF, . et al. Gabapentin for chronic neuropathic pain in adults. Cochrane Database Syst Rev. 2017; 6: CD007938. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78. Nelson JC. Tricyclic and tetracyclic drugs. : Tricyclic and Tetracyclic Drugs. 5th ed Washington, DC: American Psychiatric Association Publishing; 2017: 263. [Google Scholar]
- 79. May TW, Korn-Merker E, Rambeck B.. Clinical pharmacokinetics of oxcarbazepine. Clin Pharmacokinet. 2003; 42 12: 1023– 1042. [DOI] [PubMed] [Google Scholar]
- 80. O'Hara K. Paediatric pharmacokinetics and drug doses. Aust Prescr. 2016; 39 6: 208– 210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81. Schwartz GJ, Muñoz A, Schneider MF, . et al. New equations to estimate GFR in children with CKD. J Am Soc Nephrol. 2009; 20 3: 629– 637. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82. Bailie GR, Mason NA.. 2013 Dialysis of Drugs. Saline, MI: Renal Pharmacy Consultants; 2013. [Google Scholar]