Fig. 1.
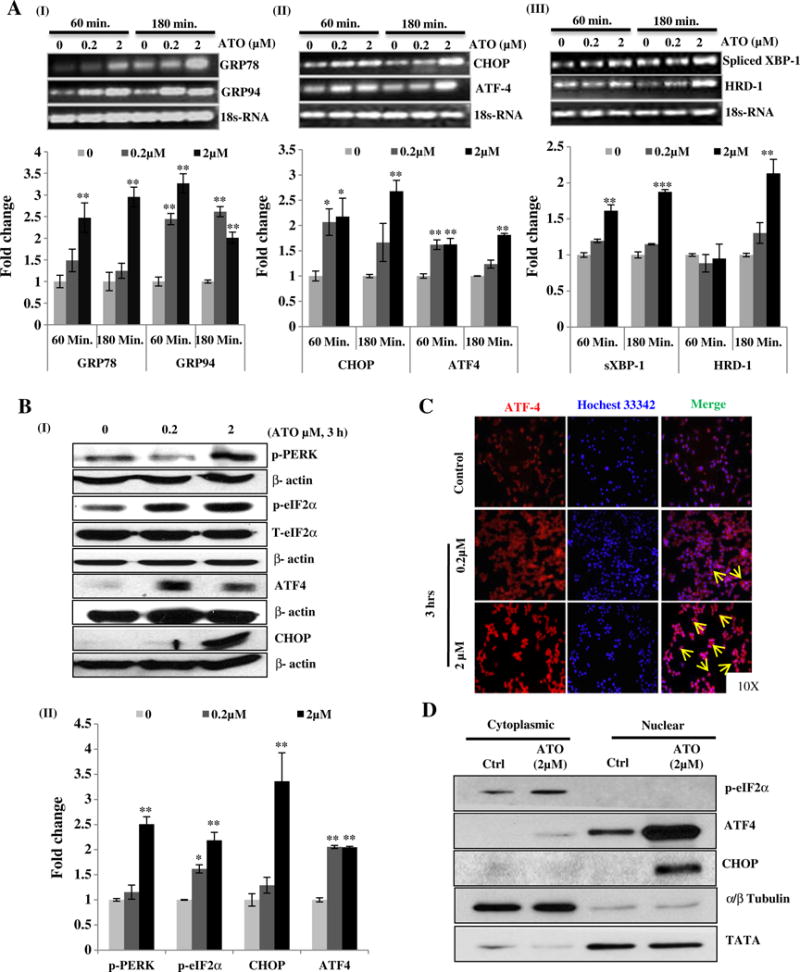
Arsenic trioxide activates UPR signaling in murine macrophage Raw 264.7 cells. Reverse transcriptase PCR analysis of UPR responsive genes in ATO-treated Raw 264.7 cells was performed. For this, cells were treated with ATO at two different concentrations (0.2 and 2 μM) for 60 and 180 min. Band intensity showing transcriptional expression levels of GRP78 and GRP94 (A-I), CHOP and ATF4 (A-II) and spliced XBP-1 and HRD1 (A-III) is depicted. Graphs indicate the densitometry analysis of band intensity in terms of fold change. 18sRNA was used as endogenous control. (B-I) Western blots analysis of UPR signaling proteins (p-PERK, p-eIF2α, ATF4 and CHOP) in ATO (0.2 and 2 μM)-treated Raw 264.7 cells for 3 h. (B-II) Relative densitometry analysis of band intensity expressed in terms of fold change. β-Actin was used as an endogenous control. (C) Immunofluorescence staining showing nuclear localization of ATF4 in ATO (0.2 and 2 μM for 3 h)-treated Raw 264.7 cells. Nuclei were stained with Hoechst 33342 dye. Arrows indicate the migration and localization of ATF4 from cytosol to nucleus in ATO-treated cells as compared to saline-treated control cells. (D) Western blot analysis of ATO-induced translocation and activation of ATF4 and its downstream molecule CHOP. Fraction purity was confirmed by α/β-tubulin and TATA binding protein for cytoplasmic and nuclear fractions respectively. Data are expressed as Mean ± SE of at least three independent samples. Statistical significance was determined using Student’s t test. *P > 0.05, **P > 0.01 and ***P > 0.001 show significance levels.
