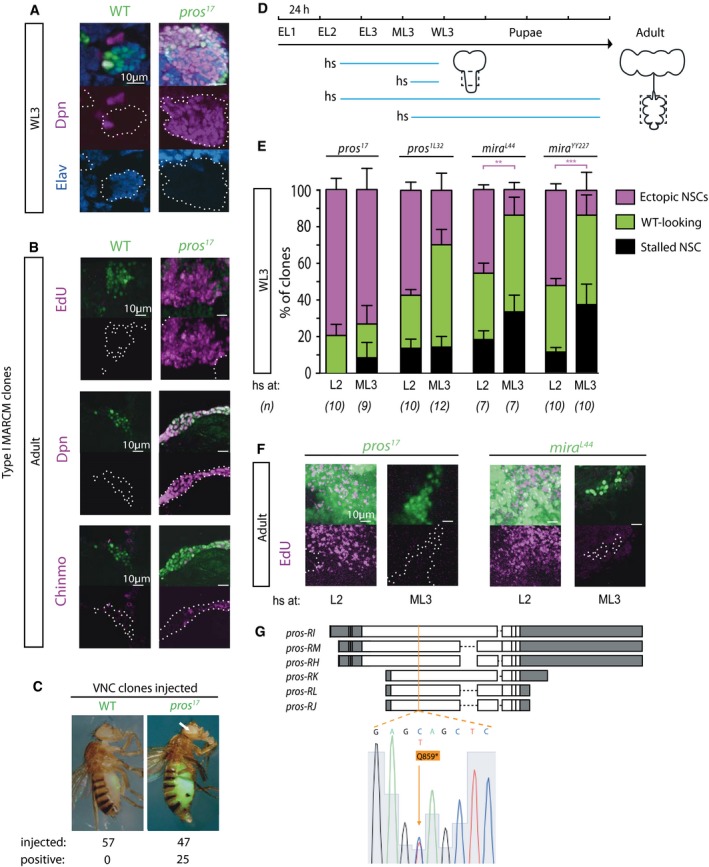
MARCM clones were induced by heat‐shock (hs) at either early L2 (EL2; “induced early”) or ML3 (“induced late”) stages and animals were allowed to develop up to either WL3 or adult stages, at which times they were dissected and incubated with EdU or fixed for immunohistochemistry. All clone pictures are single optical sections in which clones are outlined with dotted lines in split channels.
Pictures of WL3 MARCM clones induced early. Unlike WT clones, which contain a single NSC (identifiable by larger size and expression of Dpn) and mostly neurons (labeled by Elav), pros‐null clones predominantly contained Dpn+ cells and were usually devoid of neurons.
Pictures of adult MARCM clones induced early. Unlike WT clones, in which EdU, Dpn, and Chinmo are never seen at adult stages, pros‐null clones contained ectopic adult NSCs, labeled by Dpn, which were able to incorporate EdU, and including ones expressing the early temporal marker Chinmo.
Tumorigenesis assay: WL3 ventral nerve cord (VNC) fragments containing GFP‐labeled MARCM clones induced early were injected into abdomen of adult WT hosts, which resulted in invasion of the entire abdomen by pros‐null GFP+ cells in more than 50% cases (“positive”), and even detection of GFP+ cells at distant locations (arrow), which was never seen when WT GFP+ cells were injected.
Experimental design to assess tumorigenicity of pros and mira mutations induced in neural lineages at early versus late larval stages. Blue lines: developmental period between clone induction and CNS fixation. VNC clones (thoracic region outlined in dashed lines) were analyzed to ensure scoring of type I NSC clones.
WL3 clones of two independent alleles of either pros or mira were scored as: i. WT‐looking (i.e., containing a single Dpn+ cell and a few Dpn− progeny); ii. stalled NSC (i.e., containing a single Dpn+ cell and no progeny, suggesting cell‐cycle delay or stalling); iii. ectopic NSCs (i.e., containing multiple Dpn+ cells). Dpn− clones were excluded from the analysis. Histograms of clone category proportions per CNS (n = 7–12 CNSs; details in source data) show that late clone induction reduces the number of clones with ectopic NSCs in pros hypomorphic conditions such as in pros
1L32 (see (G) for molecular information) and for two mira‐null alleles [expected to behave analogously to a pros hypomorph since Mira depletion from NSCs presumably results in diminished GMC inheritance of Pros (which in some cases is followed by its reversion to NSC, hence tumorigenicity of mira mutants)]. Statistical significant difference between number of clones induced early or late (for the same genotype) that contained ectopic NSCs is indicated: **P < 0.05, ***P < 0.001 (Mann–Whitney test). Error bars SEM.
Pictures of adult MARCM clones co‐stained for EdU. Only clones induced early led to ectopic proliferation in the adult brain, indicating that ectopic NSCs visible at WL3 in clones induced late terminated proliferation prior to adult stage and are therefore non‐tumorigenic (n = 20 for each condition).
Molecular characterization of the new hypomorphic pros
1L32 allele (that survives to L1) uncovered in a forward genetic screen (R.S.N., W. Greg Somers and W. Chia, unpublished). Schematic representation of pros mRNA isoforms: Each box represents an exon; untranslated regions are in gray; and coding sequence is in white; in orange is the site of a C‐to‐T mutation—in a coding region common to all isoforms and that converts a Gln (Q) residue (position 859 in isoforms H, I, and M) to a premature STOP codon (*), resulting in severely truncated proteins.
Data information: See main text for other abbreviations.