Figure 2.
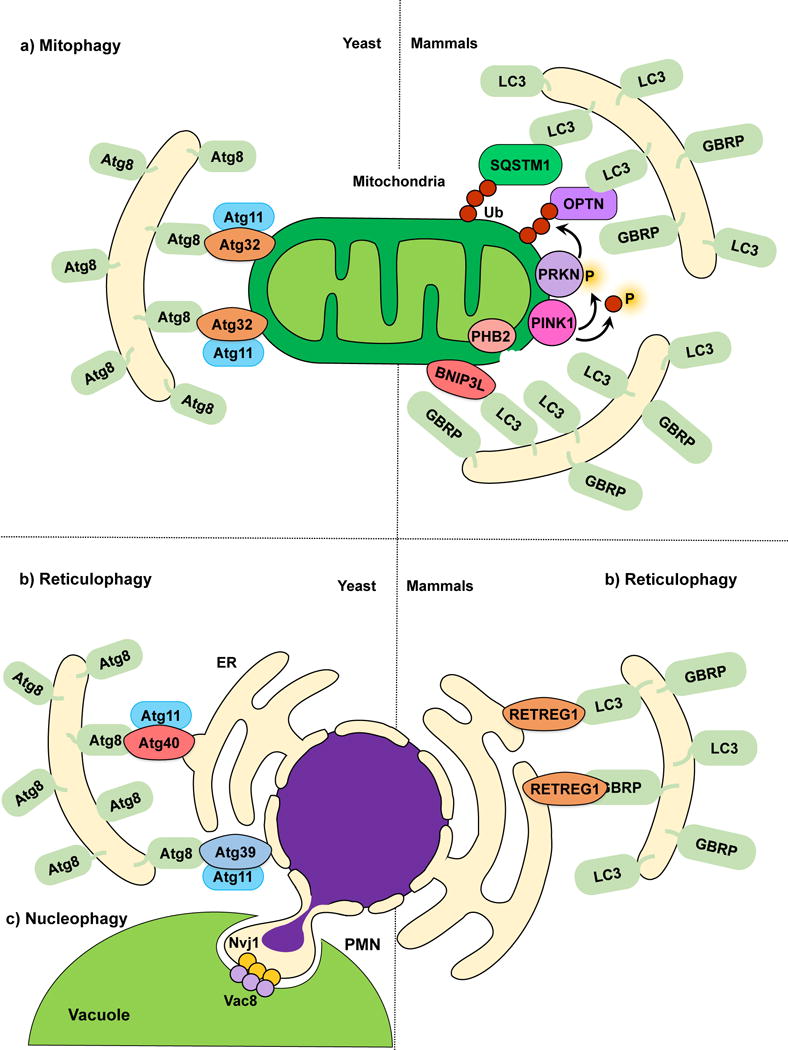
Mitophagy, reticulophagy and nucleophagy. (a) The yeast mitophagy receptor Atg32 links mitochondria to the phagophore by directly binding Atg8‒PE; Atg11 functions as a scaffold. Several cargo receptors (not all shown) have been described for mammalian mitophagy. Mitochondria depolarization leads to PINK1 activation and phosphorylation of ubiquitin and PRKN, and OMM disruption exposes PHB2. Receptors link mitochondria targeted for degradation to the phagophore. (b) In yeast reticulophagy, Atg39 and Atg40 have been proposed as receptor proteins. Atg39 mediates the degradation of the perinuclear ER, and Atg40 mediates cytoplasmic ER degradation. Both Atg39 and Atg40 link their respective ER sites to Atg8‒PE-conjugated membranes for sequestration. Atg11 has been proposed as a scaffold protein for both Atg39 and Atg40-mediated reticulophagy. During mammalian reticulophagy, RETREG1/FAM134B tethers the cytoplasmic ER to LC3/GABARAP family members for membrane sequestration and degradation. (c) Because Atg39 specifically localizes to the perinuclear ER, Atg39-mediated degradation is also considered nucleophagy. During piecemeal microautophagy of the nucleus (PMN), the nuclear envelope protein Nvj1 and vacuolar membrane protein Vac8 form nuclear-vacuolar junctions, which pinch off and engulf part of the nucleus inside the vacuole.
