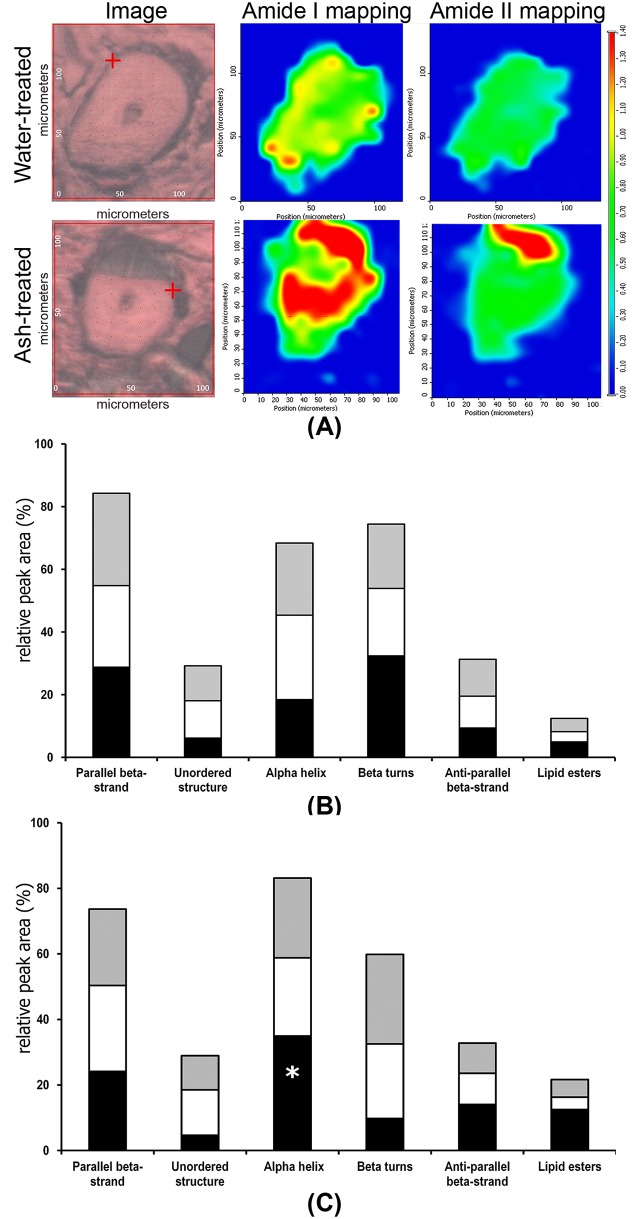
Fig 3. SR-FTIR micrographs.
(A) Micrographs and FTIR spectral images of amide I and amide II bands, derived from 128 SR-FTIR spectra of cross-sectioned grey hair samples (5 μm thick) treated with water (control) and ash extract (ash-treated); (B) and (C) relative peak areas (%) of parallel beta-strand, unordered structure, alpha-helix, beta-turns, anti-parallel beta-strand and lipid esters distributed in the cuticle (black), cortex (white) and medulla (grey) regions deconvoluted from the SR-FTIR spectra of the hair samples treated with water and ash extract, respectively. All hair samples were treated for 1 h at 25±2°C, blotted-dried and stored at 55%RH until use. (the cuticle (marked ∪), the cortex (co) and the medulla (m), * p < 0.05, compared to the control).