Abstract
Elevated CO2 and O3 can affect aphid performance via altering plant nutrients, however, little is known about the role of plant secondary metabolites in this process, especially for aphids feeding behaviors. We determined the effects of elevated CO2 and O3 on the growth and phenolics of alfalfa (Medicago sativa) and feeding behaviors of the pea aphids (Acyrthosiphon pisum) and cowpea aphids (Aphis craccivora). Elevated CO2 improved plant growth, but could not completely offset the negative effects of elevated O3. Elevated O3 increased foliar genistin content at the vegetative stage, increased ferulic acid at the reproductive stage, and elevated CO2 increased those at both stages. Simultaneously elevated CO2 and O3 increased foliar ferulic acid content at the reproductive stage and increased genistin content at both stages. For pea aphids, feeding efficiency was reduced under elevated CO2 at the reproductive stage and decreased under elevated O3 at the vegetative stage. For cowpea aphids, feeding efficiency was increased under elevated CO2 at the vegetative stage and decreased under elevated O3 at both stages. Simultaneously elevated CO2 and O3 decreased both aphids feeding efficiency. We concluded that CO2 and O3 independently or interactively had different effects on two aphids feeding behaviors through altering foliar ferulic acid and genistin contents.
Introduction
Global atmospheric concentrations of greenhouse gases (e.g., CO2 and O3) have increased due to human activities since industrialization. The CO2 concentration has increased from 280 μL/L to approximately 400 μL/L in 2017 (https://www.co2.earth/), and the tropospheric O3 concentration has increased from 10 nL/L to 50 nL/L in 20091. Furthermore, the concentrations of CO2 and O3 are expected to continue to increase2. Increases in CO2 and O3 concentrations have been anticipated to greatly influence agricultural and forest ecosystems3,4; they can directly affect plant growth, primary and secondary metabolisms, and indirectly alter interactions between plants and herbivorous insects5–7.
Elevated CO2 typically stimulates plant growth, decreases plant nitrogen concentrations, and increases the carbon:nitrogen (C:N) ratio4,8. Conversely, O3, as an oxidizing agent, enters the leaf interior through the stomata and causes leaf tissue damage, thereby inhibiting plant growth9; but the responses of foliar nutrients to elevated O3 are species-specific and depend on the duration of O3 exposure10. Elevated CO2 and O3 generally increase plant secondary metabolites, such as phenolics, including total phenolics, condensed tannins, and flavonoids6,7,11, and they also interactively affect plant metabolism that elevated CO2 tends to offset the induction of phenolics by elevated O36,12. In addition, the plant chemical composition and concentrations often change with ontogenetic stage13. For example, phenolic acids, i.e., trans-2-hydroxycinnamic, rosmarinic, vanillic, chlorogenic, gallic, and cinnamic acids, dominate during the early vegetative stage; whereas flavonoids, including amentoflavone, apigenin, quercetin, luteolin, coumarin, and rutin, predominate during the other growth stages in sweet marjoram (Origanum majorana)14. Furthermore, the concentrations of glucosinolates in Arabidopsis thaliana and phenolic glycoside in trembling aspen (Populus tremuloides) are higher in the younger leaves than in the older leaves15,16. The plant ontogenetic stage and climate changes may interactively influence plant secondary metabolites, for example, phenolic glycoside concentrations increase in the leaves of the younger trees but decrease in the older trees under elevated CO2, while elevated O3 has the opposite effects17. These changes in plant primary and secondary metabolites under elevated CO2 and O3 in turn influence the performance of insect herbivores6,7.
Many previous work has shown that elevated CO2 or O3 reduce the performance of most leaf-chewing insects due to decreased nitrogen concentrations in plants7,18,19. On the other hand, the increased concentration of plant secondary metabolites may partially explain the rduced performance of leaf-chewing insects under elevated CO2 and O3, because the secondary compounds can induce or decline the detoxication activities of the digestion system, which thereby prolonging developmental time and reducing growth rates20,21. For the piercing-sucking insects e.g., aphids, many studies have shown that elevated CO2 or O3 can increase aphid performance via promoting plant nitrogen based nutrition22–25. However, Johnson et al.26 find that the fecundity of the pea aphid (Acyrthosiphon pisum) responds differently to elevated CO2 when fed on five different alfalfa (Medicago sativa) cultures. Furthermore, the population abundance of Rhopalosiphum padi is reduced under elevated CO2 when fed on tall fescue (Schedonorus arundinaceus), but is increased when fed on barley (Hordeum vulgare)27. These results presumably indicate that the same aphid species that fed on different host plants exhibits different responses to climate changes. On the other hand, different aphid species or even genotypes that fed on the same host plants may also perform differentially under elevated CO2 and O328. For example, the specialist Brevicoryne brassicae are larger and accumulate more fat, while no changes are found in the generalist Myzus persicae reared on Brassica oleracea under elevated CO229. Therefore, the responses of aphids to elevated CO2 and O3 seem to be species-specific, demonstrating decreased, increased, or unchanged population abundance, growth, and fecundity30–33, but more evidence is needed to explain the heterogeneous responses.
Plant secondary metabolites may also contribute to the idiosyncratic responses of aphids to climate changes, though few studies have focused on it34,35. For the aphids that feed exclusively on the phloem sap, plant secondary metabolites mainly affect aphid feeding behaivor rather than the digestion system because the secondary metabolites rarely distribute in the phloem sap36,37. Many plant secondary compounds, including alkaloid, luteolin, genistein, apigenin, and saponin etc., can negatively affect the penetration pathway stage of aphid feeding38–41. For example, caffeic, ferulic, and sinapic acids disfavor the grain aphid (Sitobion avenae) feeding by prolonging the early pathway phases of probing, increasing the number of probing, and reducing salivation into sieve elements and ingestion of phloem sap42. Furthermore, the same secondary metabolites seem to have different effects on feeding activities of different aphid species. For example, the total time of probing of Aphis fabae, Aphis craccivora, A. pisum, and M. persicae increases with a reduced alkaloid content in narrow-leafed lupins (Lupinus angustifolius), whereas the alkaloid content has no influence on Macrosiphum albifrons; furthermore, when fed on the alkaloid-rich cultivar ‘Azuro’, a reduced occurrence of phloem phases is observed, especially for A. pisum and A. fabae, whereas M. albifrons shows the longest phloem phase38. However, it is also reported that secondary compounds may act as probing stimulants of aphids43. Therefore, the increases in plant secondary metabolites may increase or decrease epidermis and mesophyll resistance against aphids during pathway and probing feeding stages under elevated CO2 or O3, and the differential feeding responses of aphid species to plant secondary metabolites might contribute to their idiosyncratic responses to elevated CO2 or O3. However, the evidence about how climate changes, especially for elevated O3 alter the aphid feeding activities via plant secondary metabolites is lacking.
The current study aimed to investigate how elevated CO2 and O3, alone or in combination, alter plant secondary metabolites at different ontogenetic stages and produce cascading effects on aphid feeding behaviors. By using 12 field open-top chambers with four treatments (control, elevated CO2, elevated O3, and elevated CO2 and O3), we measured plant growth traits, secondary metabolites, and feeding behaviors of the pea aphid (Acyrthosiphon pisum Harris) and the cowpea aphid (Aphis craccivora Koch) on alfalfa (Medicago sativa). Our specific objectives were to determine: (1) the effects of elevated CO2 and O3 on plant growth traits and secondary metabolites phenolics of alfalfa, and (2) the feeding behaviors of the two species of aphids when fed on alfalfa grown under different treatments.
Results
Responses of plant growth traits to elevated CO2 and O3
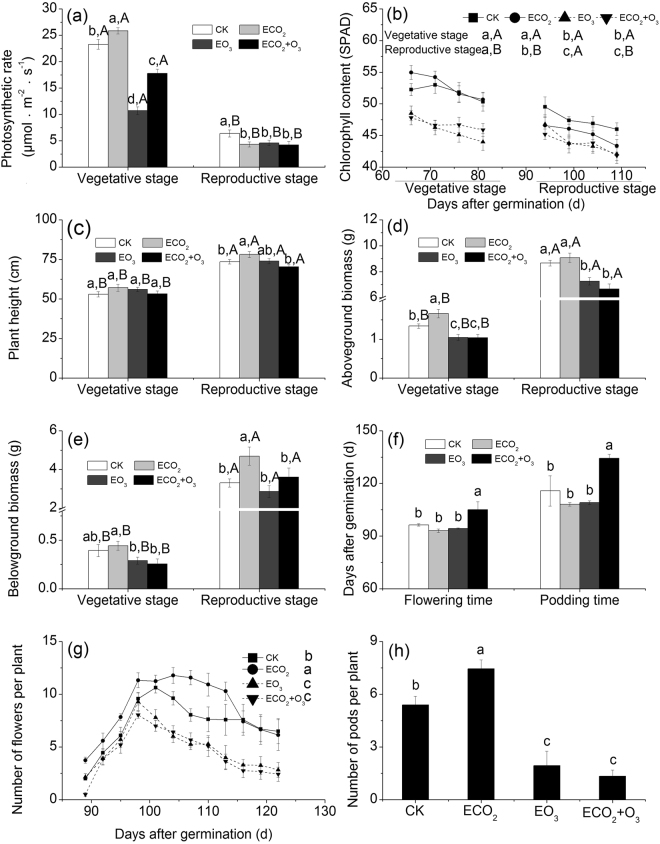
Elevated CO2, O3, plant developmental stage, and their interactions significantly influenced alfalfa growth (Table 1). Elevated CO2 did not significantly alter the plant chlorophyll content but did increase the plant net photosynthetic rate at the vegetative stage, while decreased them at the reproductive stage (significant CO2 × developmental stage interaction, Table 1, Fig. 1a,b). Elevated CO2 increased plant aboveground biomass only at the vegetative stage (Fig. 1d) and increased plant belowground biomass only at the reproductive stage (Fig. 1e). Elevated CO2 also increased the numbers of flowers and pods (Fig. 1g,h). Contrary to elevated CO2, O3 fumigation decreased the plant chlorophyll content, net photosynthetic rate, and aboveground biomass at both developmental stages, and decreased plant belowground biomass at the vegetative stage, regardless of CO2 levels (Table 1, Fig. 1a,b,d and e). Furthermore, O3 fumigation alone or in combination with CO2, also decreased the numbers of flowers and pods (Fig. 1g,h). Neither elevated CO2 nor elevated O3 affected plant flowering and podding time, though simultaneously elevated CO2 and O3 significantly delayed both (Table 1, Fig. 1f). The plant height was only significantly influenced by developmental stage, with significantly higher plant height at the reproductive stage than that at the vegetative stage (Table 1, Fig. 1c).
Table 1.
Effects of CO2, O3, plant developmental stage, and their interactions on alfalfa growth traits. F and P values from MANOVAs are shown.
| Plant growth traits | CO2 | O3 | Developmental stage | CO2 × O3 | CO2 × developmental stage | O3 × developmental stage | CO2 × O3 × developmental stage | |||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| F | P | F | P | F | P | F | P | F | P | F | P | F | P | |
| Photosynthetic rate | 12.416 | 0.001 | 122.083 | <0.001 | 807.924 | <0.001 | 17.667 | <0.001 | 34.458 | <0.001 | 85.638 | <0.001 | 3.216 | 0.076 |
| Chlorophyll content | 0.070 | 0.792 | 19.599 | <0.001 | 25.991 | <0.001 | 1.801 | 0.182 | 2.446 | 0.120 | 2.658 | 0.105 | 0.121 | 0.728 |
| Plant height | 0.913 | 0.341 | 1.006 | 0.317 | 218.391 | <0.001 | 11.098 | 0.001 | 0.002 | 0.966 | 2.680 | 0.104 | 0.003 | 0.958 |
| Aboveground biomass | 0.001 | 0.975 | 50.332 | <0.001 | 1477.290 | <0.001 | 3.541 | 0.062 | 0.418 | 0.519 | 18.135 | <0.001 | 1.124 | 0.291 |
| Belowground biomass | 8.674 | 0.004 | 7.223 | 0.008 | 296.733 | <0.001 | 0.664 | 0.417 | 7.778 | 0.006 | 3.179 | 0.077 | 0.358 | 0.551 |
| Flowering time | 2.301 | 0.180 | 4.279 | 0.084 | 8.387 | 0.027 | ||||||||
| Podding time | 0.521 | 0.497 | 0.646 | 0.452 | 1.819 | 0.226 | ||||||||
| Number of flowers per plant | 4.126 | 0.045 | 50.080 | <0.001 | 1.893 | 0.172 | ||||||||
| Number of pods per plant | 4.109 | 0.047 | 168.686 | <0.001 | 13.533 | <0.001 | ||||||||
P values < 0.05 are bolded.
Figure 1.
Growth traits of alfalfa grown under ambient or elevated CO2 and O3. (a) Photosynthetic rate, (b) Chlorophyll content, (c) Plant height, (d) Aboveground biomass, (e) Belowground biomass, (f) Flowering time and podding time, (g) Number of flowers per plant, and (h) Number of pods per plant. Values are the mean (±SE) of three replicates. Different lowercase letters indicate significant differences among the CO2 and O3 treatments within the same plant developmental stage. Different uppercase letters indicate significant differences between developmental stages within the same CO2 and O3 concentrations.
Responses of plant secondary metabolites to elevated CO2 and O3
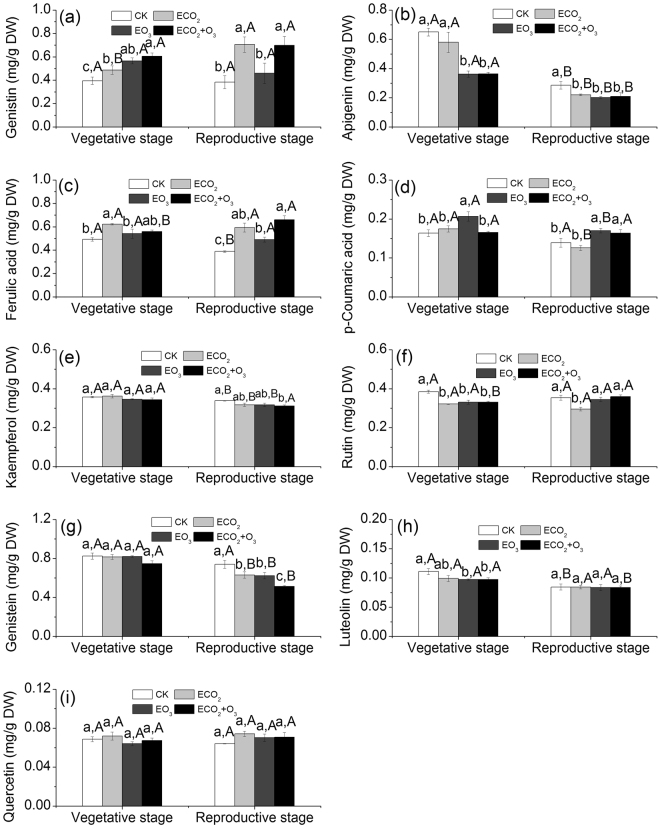
Elevated CO2 significantly influenced foliar rutin, genistein, ferulic acid, and genistin concentrations (Table 2). Elevated O3 significantly affected foliar kaempferol, apigenin, genistein, p-coumaric acid, ferulic acid, and genistin contents (Table 2). Elevated CO2 alone increased foliar ferulic acid content at both developmental stages, and elevated O3 alone or in combination with CO2 increased it only at the reproductive stage (significant CO2 × developmental stage interaction, significant O3 × developmental stage interaction, Table 2, Fig. 2c). Elevated O3 alone increased foliar p-coumaric acid content at the vegetative stage, and increased it at the reproductive stage, regardless of CO2 levels (Table 2, Fig. 2d). CO2 and O3 fumigation increased foliar genistin content at the vegetative stage, but only CO2 fumigation increased genistin content at the reproductive stage (Fig. 2a). CO2 and O3 fumigation decreased foliar apigenin content at both developmental stages, with higher values at the vegetative stage than those at the reproductive stage (Table 2, Fig. 2b). Both gases fumigation decreased foliar rutin content only at the vegetative stage and decreased genistein content only at the reproductive stage (significant CO2 × developmental stage interaction, significant O3 × developmental stage interaction, Table 2, Fig. 2f,g). Overall, elevated CO2 and O3 had significant effects on alfalfa foliar secondary metabolites, and these effects varied between vegetative stage and reproductive stage.
Table 2.
Effects of CO2, O3, plant developmental stage, and their interactions on alfalfa foliar secondary metabolite contents. F and P values from MANOVAs are shown.
| Secondary metabolites | CO2 | O3 | Developmental stage | CO2 × O3 | CO2 × developmental stage | O3 × developmental stage | CO2 × O3 × developmental stage | |||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| F | P | F | P | F | P | F | P | F | P | F | P | F | P | |
| Kaempferol | 1.666 | 0.218 | 8.618 | 0.011 | 40.404 | <0.001 | 0.162 | 0.693 | 2.099 | 0.169 | 0.003 | 0.954 | 1.393 | 0.257 |
| Rutin | 7.512 | 0.016 | 4.154 | 0.061 | 1.049 | 0.323 | 49.999 | <0.001 | 6.010 | 0.028 | 31.700 | <0.001 | 3.883 | 0.069 |
| Apigenin | 2.688 | 0.123 | 56.624 | <0.001 | 170.928 | <0.001 | 3.268 | 0.092 | 0.016 | 0.901 | 26.736 | <0.001 | 0.002 | 0.967 |
| Genistein | 19.466 | 0.001 | 19.698 | 0.001 | 103.622 | <0.001 | 0.975 | 0.340 | 3.902 | 0.068 | 5.470 | 0.035 | 0.840 | 0.375 |
| Luteolin | 1.302 | 0.273 | 2.615 | 0.128 | 44.409 | <0.001 | 1.362 | 0.263 | 1.288 | 0.276 | 1.662 | 0.218 | 1.230 | 0.286 |
| Quercetin | 3.285 | 0.091 | 0.475 | 0.502 | 0.577 | 0.460 | 0.986 | 0.338 | 0.221 | 0.646 | 1.627 | 0.223 | 1.043 | 0.325 |
| p-Coumaric acid | 4.403 | 0.054 | 16.082 | 0.001 | 19.018 | 0.001 | 4.544 | 0.051 | 0.516 | 0.485 | 2.102 | 0.169 | 6.218 | 0.026 |
| Ferulic acid | 50.654 | <0.001 | 4.673 | 0.048 | 1.053 | 0.322 | 3.631 | 0.077 | 9.938 | 0.007 | 6.503 | 0.023 | 1.288 | 0.275 |
| Genistin | 18.965 | 0.001 | 5.042 | 0.041 | 1.519 | 0.238 | 0.713 | 0.413 | 7.363 | 0.017 | 1.916 | 0.188 | 0.035 | 0.854 |
P values < 0.05 are bolded.
Figure 2.
Foliar secondary metabolites contents in alfalfa grown under ambient or elevated CO2 and O3. (a) Genistin, (b) Apigenin, (c) Ferulic acid, (d) p-coumaric acid, (e) Kaempferol, (f) Rutin, (g) Genistein, (h) Luteolin, and (i) Quercetin. Each value represents the average (±SE) of three replicates. Different lowercase letters indicate significant differences among the CO2 and O3 treatments within the same plant developmental stage. Different uppercase letters indicate significant differences between developmental stages within the same CO2 and O3 concentrations.
Responses of aphid feeding behaviors to elevated CO2 and O3
Elevated CO2 had significant effects on the time the pea aphids spent on nonpenetration, phloem feeding, potential drops, time to first potential drops, and time to first phloem feeding. Elevated O3 and developmental stage had effects similar to those for elevated CO2, except for phloem feeding (Table 3). Elevated CO2 had no significant impacts on the time the pea aphids spent on phloem feeding at the vegetative stage but decreased it at the reproductive stage, whereas elevated O3 had the opposite effects (Fig. 3a). Simultaneously elevated CO2 and O3 reduced the feeding efficiency of the pea aphids at both developmental stages (Fig. 3a), especially at the vegetative stage, simultaneously elevated CO2 and O3 increased the time to first potential drops and time to first phloem feeding (Fig. 3e,g).
Table 3.
Effects of CO2, O3, plant developmental stage, and their interactions on aphid feeding activities. F and P values from MANOVAs are shown.
| Aphids feeding activities | CO2 | O3 | Developmental stage | CO2 × O3 | CO2 × developmental stage | O3 × developmental stage | CO2 × O3 × developmental stage | |||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| F | P | F | P | F | P | F | P | F | P | F | P | F | P | |
| Pea aphid | ||||||||||||||
| np | 10.507 | 0.002 | 6.724 | 0.011 | 13.375 | <0.001 | 2.930 | 0.090 | 0.126 | 0.723 | 1.256 | 0.265 | 0.292 | 0.590 |
| C | 0.025 | 0.876 | 0.213 | 0.645 | 7.913 | 0.006 | 0.101 | 0.752 | 11.749 | 0.001 | 2.774 | 0.098 | 7.721 | 0.006 |
| E | 12.509 | 0.001 | 2.149 | 0.147 | 3.335 | 0.072 | 0.002 | 0.966 | 8.202 | 0.005 | 2.412 | 0.124 | 0.590 | 0.445 |
| pd | 5.386 | 0.022 | 20.220 | <0.001 | 29.310 | < 0.001 | 0.075 | 0.785 | 4.199 | 0.043 | 22.566 | <0.001 | 2.327 | 0.130 |
| Time to first pd | 9.641 | 0.002 | 13.297 | <0.001 | 112.430 | <0.001 | 1.474 | 0.227 | 4.104 | 0.045 | 38.395 | <0.001 | 0.064 | 0.801 |
| Time to first E | 4.162 | 0.044 | 8.828 | 0.004 | 3.675 | 0.058 | 2.397 | 0.125 | 1.936 | 0.167 | 8.914 | 0.004 | 0.866 | 0.354 |
| Total number of pd | 7.001 | 0.009 | 0.029 | 0.864 | 15.048 | <0.001 | 2.484 | 0.117 | 22.365 | <0.001 | 7.362 | 0.008 | 0.367 | 0.546 |
| Cowpea aphid | ||||||||||||||
| np | 0.057 | 0.812 | 12.406 | 0.001 | 24.891 | <0.001 | 0.385 | 0.536 | 0.666 | 0.416 | 4.234 | 0.042 | 0.013 | 0.911 |
| C | 1.696 | 0.195 | 5.275 | 0.023 | 5.207 | 0.024 | 5.395 | 0.022 | 2.644 | 0.106 | 0.027 | 0.870 | 0.610 | 0.436 |
| E | 3.498 | 0.065 | 64.482 | 0.000 | 157.132 | <0.001 | 3.424 | 0.067 | 1.846 | 0.178 | 0.152 | 0.698 | 1.850 | 0.177 |
| pd | 4.510 | 0.035 | 0.002 | 0.963 | 16.261 | <0.001 | 1.373 | 0.243 | 0.485 | 0.487 | 0.185 | 0.668 | 1.395 | 0.239 |
| Time to first pd | 4.199 | 0.043 | 53.929 | <0.001 | 18.124 | <0.001 | 0.014 | 0.907 | 2.026 | 0.158 | 13.407 | <0.001 | 18.255 | <0.001 |
| Time to first E | 2.531 | 0.114 | 19.853 | <0.001 | 3.129 | 0.080 | 1.036 | 0.311 | 0.032 | 0.859 | 0.088 | 0.767 | 1.271 | 0.262 |
| Total number of pd | 0.218 | 0.641 | 5.630 | 0.019 | 12.544 | 0.001 | 2.198 | 0.141 | 0.948 | 0.332 | 0.935 | 0.335 | 0.586 | 0.445 |
P values < 0.05 are bolded. np (nonpenetration), stylets are outside the plants; C (pathway), mostly intramural probing activities between mesophyll or parenchyma cells; E (phloem feeding), aphids are injecting watery saliva into the sieve element and ingesting the phloem sap; pd (potential drops), aphids briefly puncture cells during plant penetration.
Figure 3.
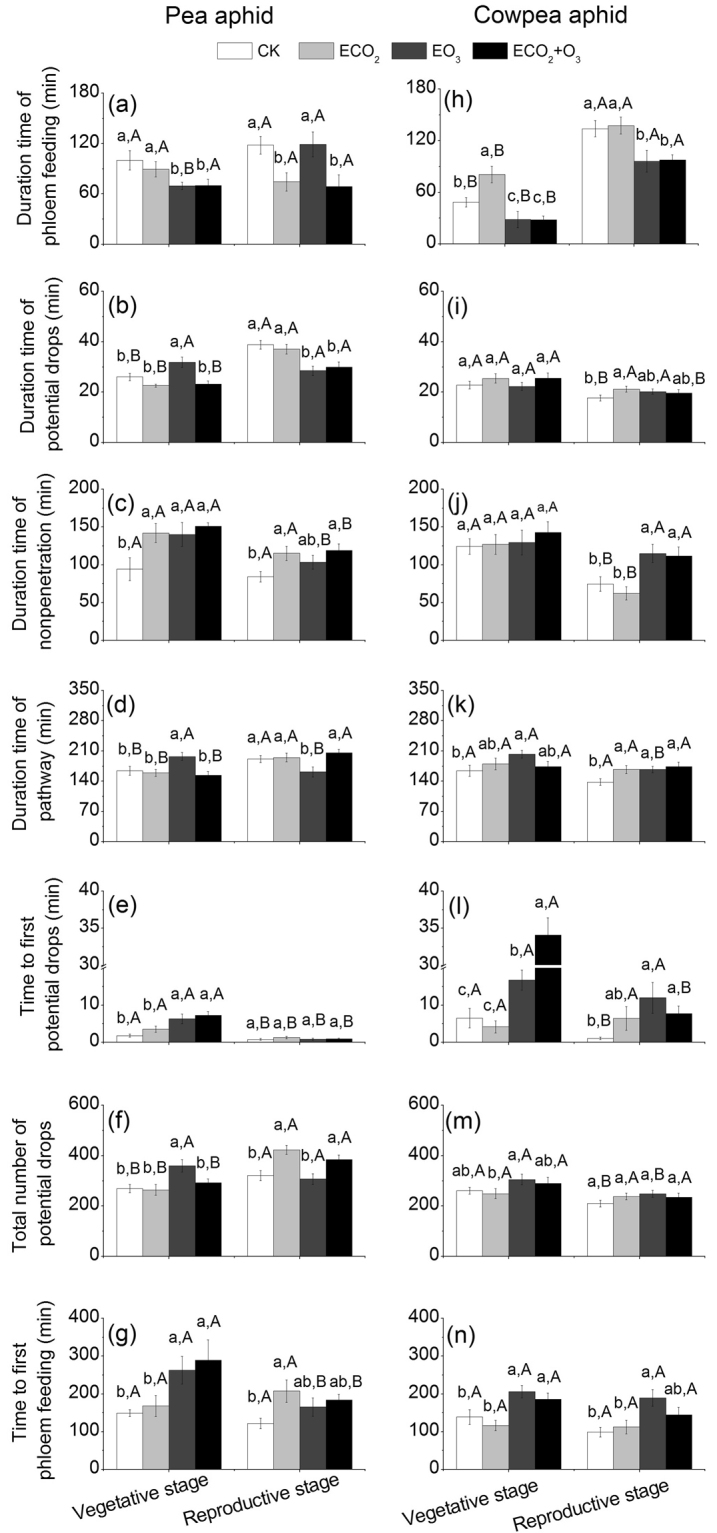
The time pea aphids and cowpea aphids spent in various feeding activities on alfalfa grown under ambient or elevated CO2 and O3. (a–g) Various feeding activities of pea aphids, (h–n) Various feeding activities of cowpea aphids. ‘Phloem feeding’ indicates that aphids are injecting watery saliva into the sieve element and ingesting the phloem sap; ‘potential drops’ indicates that aphids briefly puncture cells during plant penetration; ‘nonpenetration’ indicates that stylets are outside the plants; ‘pathway’ indicates that mostly intramural probing activities between mesophyll or parenchyma cells. Values are the means (±SE) of 21 biological replicates. Different lowercase letters indicate significant differences among the CO2 and O3 treatments within the same plant developmental stage. Different uppercase letters indicate significant differences between developmental stages within the same CO2 and O3 concentrations.
Elevated CO2 had little effect on the cowpea aphids feeding, but elevated O3 and developmental stage had significantly altered their feeding activities (Table 3). Elevated CO2 increased the time the cowpea aphids spent on phloem feeding at the vegetative stage, but had no effects at the reproductive stage (Fig. 3h). Elevated O3, alone or in combination with CO2, decreased the time the cowpea aphids spent on phloem feeding at both developmental stages (Fig. 3h). O3 fumigation also increased the time to first potential drops and time to first phloem feeding of the cowpea aphids at both developmental stages, regardless of CO2 levels (Fig. 3l,n).
Relationships between plant secondary metabolites and aphid feeding activities
Based on the aforementioned results, we calculated correlations between plant secondary metabolites that were significantly altered by CO2 and O3, and aphids feeding time of phloem feeding and potential drops. The results from the correlation analysis showed that the foliar apigenin and p-coumaric acid contents were significantly negatively correlated with the duration time of potential drops of the pea aphids, whereas the foliar ferulic acid and genistin contents were negatively correlated with the duration time of phloem feeding of the pea aphids (Table 4). The foliar p-coumaric acid content was significantly negatively correlated with the duration time of phloem feeding of the cowpea aphids, and ferulic acid content was positively correlated with the the duration time of potential drops of the cowpea aphids (Table 4).
Table 4.
Relationships between secondary metabolite contents in the leaves of alfalfa and the time aphids spent on E (phloem feeding) and pd (potential drops). Correlation coefficient r and P values are shown.
| Secondary metabolites | Pea aphid | Cowpea aphid | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| E | pd | E | pd | |||||||||
| N | r | P | N | r | P | N | r | P | N | r | P | |
| Rutin | 8 | 0.167 | 0.693 | 8 | −0.356 | 0.387 | 8 | −0.121 | 0.776 | 8 | −0.352 | 0.392 |
| Apigenin | 8 | −0.054 | 0.900 | 6 | −0.819 | 0.046 | 8 | −0.510 | 0.197 | 8 | 0.623 | 0.099 |
| Genistein | 8 | 0.108 | 0.800 | 8 | −0.050 | 0.905 | 8 | −0.552 | 0.156 | 8 | 0.531 | 0.176 |
| p-Coumaric acid | 8 | −0.232 | 0.580 | 7 | −0.867 | 0.012 | 8 | −0.781 | 0.022 | 8 | 0.388 | 0.342 |
| Ferulic acid | 8 | −0.772 | 0.025 | 8 | −0.584 | 0.128 | 8 | −0.134 | 0.752 | 8 | 0.785 | 0.037 |
| Genistin | 8 | −0.837 | 0.010 | 8 | −0.118 | 0.780 | 8 | 0.066 | 0.877 | 8 | 0.105 | 0.805 |
Values in bold indicate a significant correlation. E (phloem feeding) indicates that aphids are injecting watery saliva into the sieve element and ingesting the phloem sap; pd (potential drops) indicates that aphids briefly puncture cells during plant penetration.
Effects of secondary compounds on aphid feeding behaviors
The time the pea aphids spent on phloem feeding was significantly reduced on treated plants but not on +ferulic acid compared to controls (Fig. 4a). The time that the pea aphids spent on potential drops was significantly increased, and the total number of potential drops was significantly increased on the plants treated with +apigenin and +p-coumaric acid (Fig. 4b,f).
Figure 4.
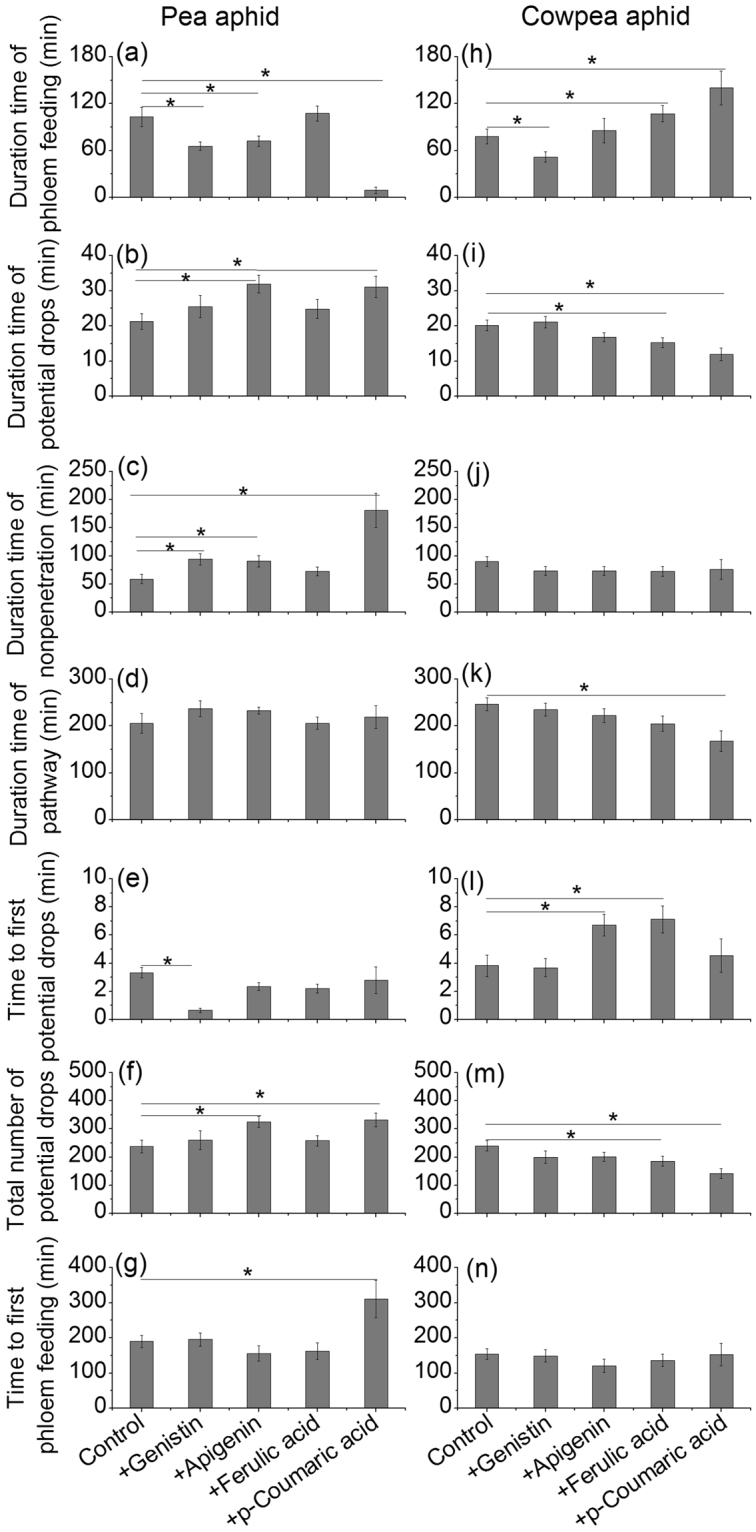
The time pea aphids and cowpea aphids spent in various feeding activities on treated (+genistin, +apigenin, +ferulic acid, and +p-coumaric acid) and control plants. (a–g) Various feeding activities of pea aphids, (h–n) Various feeding activities of cowpea aphids. ‘Phloem feeding’ indicates that aphids are injecting watery saliva into the sieve element and ingesting the phloem sap; ‘potential drops’ indicates that aphids briefly puncture cells during plant penetration; ‘nonpenetration’ indicates that stylets are outside the plants; ‘pathway’ indicates that mostly intramural probing activities between mesophyll or parenchyma cells. Values are the means (±SE) of 15–20 biological replicates. Significant differences: *there was significant difference between the treatment and control at P < 0.05.
Supplemental genistin also decreased the time the cowpea aphids spent on phloem feeding, whereas +ferulic acid and +p-coumaric acid significantly increased it (Fig. 4h). In addition, +ferulic acid and +p-coumaric acid reduced the time the cowpea aphids spent on potential drops and the total number of potential drops (Fig. 4i,m).
Discussion
Elevated CO2 and O3 affect the performance of herbivorous insects mainly by altering host plant primary and secondary metabolites6. Phenolics are important secondary metabolites for plants in defence against herbivory44. In the current study, elevated CO2 and O3 altered alfalfa growth traits and concentrations of phenolics such as genistin, ferulic acid, p-coumaric acid, and apigenin, which were antifeedants or feeding stimuli to aphids according to our exogenous application bioassay. Thus, aphid feeding efficiency was differentially altered by these changes in secondary metabolites. Furthermore, the responses of plant secondary metabolites and corresponding aphid feeding activities to elevated CO2 and O3 varied between plant developmental stages.
The positive effects of elevated CO2 on the plant photosynthesis, growth, and seed yield in legume alfalfa are consistent with the results of a previous work that has studied soybean (Glycine max)45. However, elevated O3 negatively affected alfalfa growth, and these reductions in plant photosynthesis and growth under elevated O3 may be due to the generation of ROS damage to photosynthetic processes such as the synthesis of rubisco46. Our study also showed that elevated CO2 did not offset the negative effects of elevated O3 on the plant growth. This finding is in contrast to other studies in which elevated CO2 has been shown to ameliorate the negative effects of elevated O347,48. These contradictory results may be due to the interactions between CO2 and O3 depending on plant species49 and plant developmental stages17.
In addition to having effects on alfalfa growth traits, elevated CO2 and O3 altered the concentrations of the foliar secondary metabolites phenolics, such as rutin, ferulic acid, genistin, apigenin, and p-coumaric acid. For example, ferulic acid and genistin contents were significantly increased at both plant developmental stages under elevated CO2. Plant phenolics are formed from phenylalanine via the shikimic acid-phenylpropanoid pathway50, and the biosynthesis of phenylpropanoids requires the efficient flow of carbon into phenylalanine biosynthesis51. This process can be regulated by phytohormones, such as JA (jasmonic acid), SA (salicylic acid), and ET (ethylene), which can be affected by elevated CO252,53. For example, elevated CO2 increases the concentration of SA-regulated phenolics, such as flavonoids (e.g., quercetin, kaempferol, and fisetin) in Malaysian young ginger (Zingiber officinale Roscoe)54, but reduces the concentration of JA-regulated isoflavonoids, such as genistein in soybean (G. max)55. Although our results are not absolutely consistent with these studies mentioned above, we all indicate that individual phenolic compounds differentially respond to elevated CO2. In addition, plant secondary metabolites, such as alkaloids and phenolics, often change dramatically as plants develop56,57. Furthermore, plant developmental stages interact with atmospheric changes to influence the secondary metabolism6. However, most studies have investigated only one developmental stage. For example, six of the nine sympatric British grassland species studied exhibits a significant increase in one or more secondary metabolites throughout seedling ontogeny56. Few studies have focused on multiple ontogenetic stages, such as the seedling stage, vegetative juvenile stage, and mature stage58. Our study included two plant developmental stages, the vegetative and reproductive stages. We found that the responses of foliar ferulic acid and genistin contents to elevated CO2 and O3 were influenced by the plant developmental stage. For example, genistin content was increased only at the vegetative stage, ferulic acid content was increased only at the reproductive stage under elevated O3, and the foliar apigenin, kaempferol, and genistein contents were much higher at the vegetative stage than those at the reproductive stage. The heterogeneous defence chemical composition between vegetative and reproductive stages might be explained by that the direction of changes in defensive compounds during the transition from juvenile to mature stage depends on the types of compounds in herbs58. An increase in phenolic content under O3 fumigation is also commonly reported6, though elevated O3 has been shown to damage plant photosynthesis59. These changes in phenolics under elevated O3 may be due to the increase in the activities of phenylalanine-ammonium lyase (PAL) and chalcone synthase enzymes (CHS), which are key enzymes in the biosynthesis of phenolics60. The increased phenolics may also act as antioxidants against oxidative stress caused by O361.
Plant secondary metabolites can affect the behavior, growth, and development of herbivorous insects62,63. According to our bioassay, ferulic acid and p-coumaric acid acted as feeding stimulants of the cowpea aphids, while ferulic acid did not affect the pea aphids, and p-coumaric acid acted as an antifeedant of the pea aphids. These results suggest that a single compound can have different and even opposite effects on these two species of aphids. Similar results have been reported for p-coumaric acid and ferulic acid, which are phagostimulants for the stem borer (Chilo partellus Swinhoe)64, but feeding inhibitors for maize weevil (Sitophilus zeamais Motschulsky)65. However, studies have concluded that p-coumaric acid and ferulic acid have negative effects on the performance of the grain aphid (S. avenae)66. The discrepancy among these studies might be due to the feeding guilds or food habits of herbivores, such as specialist and generalist responding differently to plant secondary chemistry67–69, reflecting different coevolution between insects and plant defence. In this study, genistin acted as an antifeedant of both the pea aphids and cowpea aphids, which had been demonstrated to negatively affect stinkbugs (Nezara viridula and Piezodorus guildinii) and whitefly (Bemisia tabaci)70,71. They all seem to imply that genistin may be important secondary compounds conferring resistance to many insects that belong to different feeding guilds. Our study also indicated that p-coumaric acid did not play a key role in responses of the cowpea aphids feeding to elevated O3, though p-coumaric acid acted as their feeding stimulant. This may be because many compounds coexist in plant leaves and interactions among them may alter the effects of a single chemical62.
In contrast to studies suggesting that elevated CO2 favours aphid phloem feeding, such as pea aphid fed on Meidicago truncatula and green peach aphid fed on Nicotiana attenuata72–74, our research demonstrated that elevated CO2 discouraged the pea aphids feeding at the reproductive stage, though favoured the cowpea aphids feeding at the vegetative stage. These results imply that the responses of aphids or even aphid-plant to elevated CO2 are species-specific. The heterogeneous responses have been widely reported75, however, the underlying mechanism is very complex. Our study suggests that the differential responses of two species of aphids to various compounds (genistin, ferulic acid, and p-coumaric acid) may be one reason. Indeed, studies have also shown that the aphid species or genotypes differentially respond to the same secondary compounds, such as thymol, hydroquinone, and alkaloid38,76, but more efforts are needed to explain the heterogeneous responses of aphids to atmospheric changes.
The performance of aphids can be decreased, increased, or unchanged under elevated O3, depending on the duration and concentration of O3 exposure and the age of the exposed plants32,77. However, our results showed that elevated O3 decreased the feeding efficiency of aphids at both plant developmental stages, except for no impact on phloem feeding of the pea aphids at the reproductive stage. Furthermore, the feeding efficiency of two species of aphids was inhibited under simultaneously elevated CO2 and O3, indicating that elevated CO2 did not completely offset the negative effects of elevated O3. This finding is in contrast to previous studies that elevated CO2 ameliorates the negative impact of elevated O3 on herbivores19,78. A possible explanation is that those studies use leaf-chewing herbivores and use trees as the host plants, while we use phloem-feeding insects and use herbage as the host plants. The SoyFACE experiments have demonstrated that elevated O3 has no impacts on soybean aphid (Aphis glycine) numbers, and that the effects of simultaneously elevated CO2 and O3 on aphid are similar to that of elevated CO2 alone79. Thus, the responses of plant growth forms and feeding guilds to atmospheric changes seem to be heterogeneous.
The decreased time the pea aphids and cowpea aphids spent on phloem feeding under simultaneously elevated CO2 and O3 may result in reduced direct damage to the plants. In addition, the aphid feeding activities can also be used to predict indirect damage caused by transmitting plant virus. The piercing-sucking mouthparts of aphids facilitate the delivery of virions into plant cells without causing irrevocable damage80. Among a series of aphid feeding activities, the potential drops (pd) and phloem feeding (E) are relevant to virus transmission81. Elevated CO2 and O3 significantly influenced the time aphids spent on potential drops (pd) and the total number of potential drops (pd), which in turn altered the transmission of stylet-borne viruses by aphids. However, more evidence is needed to demonstrate how the loss of plant productivity caused by virus transmission will be altered under future atmospheric changes.
In summary, elevated CO2 and O3 have the potential to affect aphid feeding behaviors via the alteration of plant secondary metabolites, and the responses of aphids to climate changes depend on aphid species and plant developmental stage. This study has generated several significant findings. First, it provides evidence that the heterogeneous responses of aphids to atmospheric changes may result from the differential responses of aphids to the chemicals. Second, direct damage and population outbreaks of aphids may be decreased under future atmospheric conditions due to the reduced efficiency of phloem ingestion under simultaneously elevated CO2 and O3. Finally, and perhaps most importantly, the responses of aphids to elevated CO2 and O3 alone or in combination are different and vary with plant developmental stage, suggesting that multi-factor and long-term research is needed. More research is needed to further elucidate the mechanisms underlying the effects of elevated CO2 and O3 on herbivores and the role of plant secondary metabolites in the adaption of aphids to future atmospheric environments.
Methods
Treatments under different CO2 and O3 concentrations
The study was performed from March to July 2015 in 12 octagonal open-top chambers (OTCs) at the Observation Station of the Global Change Biology Group, Institute of Zoology, Chinese Academy of Sciences in Xiaotangshan County, Beijing, China (40°11′N, 116°24′E). The atmospheric CO2 and O3 concentration treatments were as follows: current atmospheric CO2 and O3 concentrations (CK, 400 μL/L CO2 and 35 nL/L O3), elevated CO2 concentration (ECO2, 750 μL/L CO2), elevated O3 concentration (EO3, 70 nL/L O3), and simultaneously elevated CO2 and O3 concentrations (ECO2 + O3, 750 μL/L CO2 and 70 nL/L O3). Three blocks were used for CO2 and O3 treatments, and each block contained four OTCs, one OTC with ambient atmospheric CO2 and O3 concentrations, one OTC with an elevated CO2 concentration, one OTC with an elevated O3 concentration, and one OTC with elevated CO2 and O3 concentrations. CO2 and O3 concentrations in each OTC were monitored and adjusted with an infrared CO2 analyser (Ventostat 8102; Telaire Company, Goleta, CA, USA) and O3 analyser (Aeroqual, series 200, New Zealand), respectively, once every minute to maintain relatively stable CO2 and O3 concentrations. The OTCs were ventilated with air daily from 8:00 am to 5:30 pm.
Aphids and host plants
The pink pea aphid (A. pisum) and the cowpea aphid (A. craccivora) were collected from alfalfa (M. sativa). The nymphal instars from the same parthenogenetic aphid female were reared on alfalfa with 14 h light (25 °C): 10 h dark (22 °C) in photoclimate chambers (Safe PRX-450C, Ningbo, China).
The alfalfa cultivar ‘Algonquin’ was purchased from the Chinese Academy of Agricultural Sciences. The ‘Algonquin’ seeds were sown in sterilized soil and watered every 4 days. After the seedlings had grown in sterilized soil for 2 weeks, they were transplanted into plastic pots (17 cm diameter and 24 cm height) and placed in the OTCs. Pot placement was re-randomized within each OTC once per week. No chemical fertilizers or insecticides were used. After the plants were fumigated for 3 weeks (vegetative stage) or 8 weeks (reproductive stage), they were used for the assays described in the following sections.
Plant growth traits
Six plants per OTC (18 plants for each treatment and 72 plants in total) were randomly selected for measurement of growth traits. The leaf chlorophyll content was determined with a Minolta SPAD-502 plus (Konica Minolta Sensing Inc., Osaka, Japan). The leaf net photosynthetic rate was determined with a Li-Cor 6400 gas exchange system (Li-Cor Inc., Lincoln, NE, USA) between 9:00 hours and 12:00 hours. These plants were observed every day for flowering and podding after they had grown for 2 months. The remaining 12 plants per OTC (six for the vegetative stage, six for the reproductive stage, and 144 plants in total) were harvested for measurement of the biomass. The shoots and roots of each plant were collected, oven-dried (50 °C) for 48 h, and weighed.
Aphid feeding behaviors
Seven plants per OTC (21 plants for each treatment and 84 plants for each aphid) were randomly selected as host plants at the vegetative stage to evaluate aphid feeding behaviors using the electrical penetration graph (EPG) technique. Another 84 plants were also randomly selected at the reproductive stage. The principle of EPG was introduced by Tjallingii and Hogen-Esch82. Eight plants were placed in a Faraday cage to avoid noise and interference. Each plant was infested with one apterous adult aphid, and its feeding behavior was recorded for 8 h. The aphids were starved for 10 h before the test. Two eight-channel amplifiers simultaneously recorded 16 individual aphids on separate plants (four plants per treatment). Twenty-one biological replicates were included for each treatment. The feeding waveforms in this study were scored according to Tjallingii and Hogen-Esch82: nonpenetration (np), stylets are outside the plants; pathway (C), mostly intramural probing activities between mesophyll or parenchyma cells; phloem feeding (E), aphids are injecting watery saliva into the sieve element and ingesting the phloem sap; potential drops (pd), aphids briefly puncture cells during plant penetration.
Plant secondary metabolites
The chemical analysis was determined according to Oleszek and Stochmal83 and Nour et al.84 with some modification. Freeze-dried leaves were ground into a fine powder. For a typical extraction, approximately 50 mg samples were soaked with 1.5 mL of 70% aqueous MeOH for 1 h in a 60 °C water bath. The extract was centrifuged at 12,000 rpm for 15 min, and the supernatant was filtered with a 0.22 μm filter. The samples were stored in a −20 °C freezer until chemical analysis. Using high-performance liquid chromatography (HPLC), we analysed 12 phenolic compounds: phenolic acids, including chlorogenic acid, caffeic acid, cinnamic acid, p-coumaric acid, and ferulic acid; flavonoids, including rutin, luteolin, apigenin, kaempferol, and quercetin; and isoflavones, including genistein and genistin. Among these compounds, chlorogenic acid, caffeic acid, and cinnamic acid were not detected in the alfalfa leaves. Determination of compounds was performed on a Waters system with a diode array detector. Chromatograms were registered and integrated at 280, 350, and 254 nm for phenolic acid, flavonoids, and isoflavone, respectively. The mobile phase consisted of 1% H3PO4-AcN (a linear gradient of 15–100% AcN) with a flow rate of 1 mL/min for 60 min. Compounds were identified by comparing retention times to those of authentic standards.
Bioassays with pure compounds
According to the chemical analysis, elevated CO2 and O3 had significant impacts on foliar rutin, genistin, ferulic acid, p-coumaric acid, genistein, and apigenin contents of alfalfa (Table 2). As aphid feeding behaviors were altered under elevated CO2 and O3, and as aphid feeding activities were significantly related to genistin, ferulic acid, p-coumaric acid, and apigenin (Table 4), we performed bioassays to test whether the four secondary compounds affected the aphid feeding. All test compounds were commercially available products.
For the bioassay, differing from the in vitro detached leaves and artificial diets73,85, we applied the pure compounds to the living plant leaves to provide aphids with the most realistic feeding conditions. All the bioassays were performed on alfalfa plants in a greenhouse. Twenty plants were randomly selected for the experiment. The leaves in the same location were separately treated with 50 μL genistin, ferulic acid, p-coumaric acid, and apigenin solution. Five biological replicates were included for each treatment. Another five plants were selected as controls. The treated and adjacent systematic control leaves were collected to measure the concentrations of the four compounds at 24 h, 48 h, and 72 h after treatment. Contents of genistin, ferulic acid, p-coumaric acid, and apigenin were much higher in the treated leaves compared to the control leaves. The variation tendency remained similar at 24 h, 48 h, and 72 h (see Supplementary Table S1 online). Thus, we selected 24 h to assess the effects of compounds on aphid feeding behaviors using EPG as above.
Statistical analysis
We analysed the univariate responses of the growth traits, secondary metabolite contents and aphid feeding activities with a split-plot design using the following model Yijkl = bi + Cj + Ok + COjk + eijk + Dl + CDjl + ODkl + CODjkl + εijkl. In this model, b represents block i = 3, C represents CO2 level j = 2, O represents O3 level k = 2, eijk represents the whole-plot error, D represents developmental stage l = 2, and εijkl represents the sub-plot error. Yijkl represents the average response of block i, CO2 level j, O3 level k and developmental stage l (SAS 9.2, USA). Effects were considered significant when P < 0.05. LSD multiple range tests were used to separate means when ANOVAs were significant.
Acknowledgements
This project was supported by the Strategic Priority Research Program of the Chinese Academy of Sciences (no. XDB11050400) and the National Nature Science Fund of China (no. 31770452).
Author Contributions
H.Y., Y.S. and F.G. formulated the original idea and developed methodology; H.Y. performed experimental processing and statistical analysis; E.Y. helped to analyze chemicals; H.Y. interpreted the data; H.Y. wrote the manuscript with editorial advice from F.G., Y.S. and H.G. All authors gave final approval for publication.
Competing Interests
The authors declare no competing interests.
Footnotes
Publisher's note: Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Contributor Information
Yucheng Sun, Email: sunyc@ioz.ac.cn.
Feng Ge, Email: gef@ioz.ac.cn.
References
- 1.Feng Z, Kobayashi K. Assessing the impacts of current and future concentrations of surface ozone on crop yield with meta-analysis. Atmo. Environ. 2009;43:1510–1519. doi: 10.1016/j.atmosenv.2008.11.033. [DOI] [Google Scholar]
- 2.Intergovernmental Panel on Climate Change (IPCC). Climate change: The physical science basis. Contribution of working group I to the fifth assessment report of the Intergovernmental Panel onClimate Change (ed. Stocker, T. F. et al.) (Cambridge University Press, 2013)
- 3.Fuhrer J. Agroecosystern responses to combinations of elevated CO2, ozone, and global climate change. Agr Ecosyst Environ. 2003;97:1–20. doi: 10.1016/S0167-8809(03)00125-7. [DOI] [Google Scholar]
- 4.Lindroth RL. Impacts of elevated atmospheric CO2 and O3 on forests: phytochemistry, trophic interactions, and ecosystem dynamics. J. Chem. Ecol. 2010;36:2–21. doi: 10.1007/s10886-009-9731-4. [DOI] [PubMed] [Google Scholar]
- 5.Ainsworth EA, Yendrek CR, Sitch S, Collins WJ, Emberson LD. The effects of troposphericozone on net primary productivity and implications for climate change. Annu Rev Plant Biol. 2012;63:637–661. doi: 10.1146/annurev-arplant-042110-103829. [DOI] [PubMed] [Google Scholar]
- 6.Valkama E, Koricheva J, Oksanen E. Effects of elevated O3, alone and in combination with elevated CO2, on tree leaf chemistry and insect herbivore performance: a meta-analysis. Global Change Biol. 2007;13:184–201. doi: 10.1111/j.1365-2486.2006.01284.x. [DOI] [Google Scholar]
- 7.Robinson EA, Ryan GD, Newman JA. A meta-analytical review of the effects of elevated CO2 on plant-arthropod interactions highlights the importance of interacting environmental and biological variables. New Phytol. 2012;194:321–336. doi: 10.1111/j.1469-8137.2012.04074.x. [DOI] [PubMed] [Google Scholar]
- 8.Ainsworth EA, Long SP. What have we learned from 15 years of free-air CO2 enrichment (FACE)? A meta-analytic review of the responses of photosynthesis, canopy. New Phytol. 2005;165:351–371. doi: 10.1111/j.1469-8137.2004.01224.x. [DOI] [PubMed] [Google Scholar]
- 9.Karnosky DF, et al. Free-air exposure systems to scale up ozone research to mature trees. Plant Biol. 2007;9:181–190. doi: 10.1055/s-2006-955915. [DOI] [PubMed] [Google Scholar]
- 10.Oksanen E. Responses of selected birch (Betula pendula Roth) clones to ozone change over time. Plant Cell Environ. 2003;26:875–886. doi: 10.1046/j.1365-3040.2003.01020.x. [DOI] [PubMed] [Google Scholar]
- 11.Fares S, Oksanen E, Lannenpaa M, Julkunen-Tiitto R, Loreto F. Volatile emissions and phenolic compound concentrations along a vertical profile of Populus nigra leaves exposed to realistic ozone concentrations. Photosynth. Res. 2010;104:61–74. doi: 10.1007/s11120-010-9549-5. [DOI] [PubMed] [Google Scholar]
- 12.Peltonen PA, Vapaavuori E, Julkunen-tiitto R. Accumulation of phenolic compounds in birch leaves is changed by elevated carbon dioxide and ozone. Global Change Biol. 2005;11:1305–1324. doi: 10.1111/j.1365-2486.2005.00979.x. [DOI] [Google Scholar]
- 13.Holeski LM, Hillstrom ML, Whitham TG, Lindroth RL. Relative importance of genetic, ontogenetic, induction, and seasonal variation in producing a multivariate defense phenotype in a foundation tree species. Oecologia. 2012;170:695–707. doi: 10.1007/s00442-012-2344-6. [DOI] [PubMed] [Google Scholar]
- 14.Sellami IH, et al. Effect of growth stage on the content and composition of the essential oil and phenolic fraction of sweet marjoram (Origanum majorana L.) Ind. Crop. Prod. 2009;30:395–402. doi: 10.1016/j.indcrop.2009.07.010. [DOI] [Google Scholar]
- 15.Brown PD, Tokuhisa JG, Reichelt M, Gershenzon J. Variation of glucosinolate accumulation among different organs and developmental stages of Arabidopsis thaliana. Phytochemistry. 2003;62:471–481. doi: 10.1016/S0031-9422(02)00549-6. [DOI] [PubMed] [Google Scholar]
- 16.Donaldson JR, Stevens MT, Barnhill HR, Lindroth RL. Age-related shifts in leaf chemistry of clonal aspen (Populus tremuloides) J. Chem. Ecol. 2006;32:1415–1429. doi: 10.1007/s10886-006-9059-2. [DOI] [PubMed] [Google Scholar]
- 17.Couture J, Holeski L, Lindroth R. Long-term exposure to elevated CO2 and O3 alters aspen foliar chemistry across developmental stages. Plant Cell Environ. 2014;37:758–765. doi: 10.1111/pce.12195. [DOI] [PubMed] [Google Scholar]
- 18.Coll M, Hughes L. Effects of elevated CO2 on an insect omnivore: A test for nutritional effects mediated by host plants and prey. Agr. Ecosyst. Environ. 2008;123:271–279. doi: 10.1016/j.agee.2007.06.003. [DOI] [Google Scholar]
- 19.Couture JJ, Lindroth RL. Atmospheric change alters performance of an invasive forest insect. Global Change Biol. 2012;18:3543–3557. doi: 10.1111/gcb.12014. [DOI] [Google Scholar]
- 20.Caballero C, Lopez-Olguin J, Ruiz M, Ortego F, Castanera P. Antifeedant activity and effects of terpenoids on detoxication enzymes of the beet armyworm, Spodoptera exigua (Hubner) Span. J. Agric. Res. 2008;6:177–184. doi: 10.5424/sjar/200806S1-386. [DOI] [Google Scholar]
- 21.Hemming JDC, Lindroth RL. Effects of phenolic glycosides and protein on gypsy moth (Lepidoptera: Lymantriidae) and forest tent caterpillar (Lepidoptera: Lasiocampidae) performance and detoxication activities. Environ. Entomol. 2000;29:1108–1115. doi: 10.1603/0046-225X-29.6.1108. [DOI] [Google Scholar]
- 22.Kainulainen P, Holopainen J, Holopainen T. Combined effects of ozone and nitrogen on secondary compounds, amino acids, and aphid performance in Scots pine. J. Environ. Qual. 2000;29:334–342. doi: 10.2134/jeq2000.00472425002900010042x. [DOI] [Google Scholar]
- 23.Guo H, et al. Pea aphid promotes amino acid metabolism both in Medicago truncatula and bacteriocytes to favor aphid population growth under elevated CO2. Global Change Biol. 2013;19:3210–3223. doi: 10.1111/gcb.12260. [DOI] [PubMed] [Google Scholar]
- 24.Guo H, et al. Elevated CO2 decreases the response of the ethylene signaling pathway in Medicago truncatula and increases the abundance of the pea aphid. New Phytol. 2014;201:279–291. doi: 10.1111/nph.12484. [DOI] [PubMed] [Google Scholar]
- 25.Dáder, B., Fereres, A., Moreno, A. & Trebicki, P. Elevated CO2 impacts bell pepper growth with consequences to Myzus persicae life history, feeding behaviour and virus transmission ability. Sci. Rep. 6 (2016). [DOI] [PMC free article] [PubMed]
- 26.Johnson S, Ryalls J, Karley A. Globalclimate change and crop resistance to aphids: contrasting responses of lucerne genotypes to elevated atmospheric carbon dioxide. Ann. Appl. Biol. 2014;165:62–72. doi: 10.1111/aab.12115. [DOI] [Google Scholar]
- 27.Ryan, G. Plant-mediated aphid responses to elevated CO2: The impact of phloem phytochemistry, endophyte infection and nitrogen availability. Doctor of Philosophy thesis, University of Guelph (2012).
- 28.Mondor EB, Tremblay MN, Awmack CS, Lindroth RL. Altered genotypic and phenotypic frequencies of aphid populations under enriched CO2 and O3 atmospheres. Global Change Biol. 2005;11:1990–1996. [Google Scholar]
- 29.Stacey DA, Fellowes MD. Influence of elevated CO2 on interspecific interactions at higher trophic levels. Global Change Biol. 2002;8:668–678. doi: 10.1046/j.1365-2486.2002.00506.x. [DOI] [Google Scholar]
- 30.Percy KE, et al. Altered performance of forest pests under atmospheres enriched by CO2 and O3. Nature. 2002;420:403–407. doi: 10.1038/nature01028. [DOI] [PubMed] [Google Scholar]
- 31.Ryan GD, Sylvester EV, Shelp BJ, Newman JA. Towards an understanding of how phloem amino acid composition shapes elevated CO2-induced changes in aphid population dynamics. Ecol. Entomol. 2015;40:247–257. doi: 10.1111/een.12181. [DOI] [Google Scholar]
- 32.Holopainen JK. Aphid response to elevated ozone and CO2. Entomol. Exp. Appl. 2002;104:137–142. doi: 10.1046/j.1570-7458.2002.01000.x. [DOI] [Google Scholar]
- 33.Awmack CS, Harrington R, Lindroth RL. Aphid individual performance may not predict population responses to elevated CO2 or O3. Global Change Biol. 2004;10:1414–1423. doi: 10.1111/j.1365-2486.2004.00800.x. [DOI] [Google Scholar]
- 34.Peltonen PA, Julkunen-Tiitto R, Vapaavuori E, Holopainen JK. Effects of elevated carbon dioxide and ozone on aphid oviposition preference and birch bud exudate phenolics. Global Change Biol. 2006;12:1670–1679. doi: 10.1111/j.1365-2486.2006.01226.x. [DOI] [Google Scholar]
- 35.Klaiber J, Najar-Rodriguez AJ, Dialer E, Dorn S. Elevated carbon dioxide impairs the performance of a specialized parasitoid of an aphid host feeding on Brassica plants. Biol Control. 2013;66:49–55. doi: 10.1016/j.biocontrol.2013.03.006. [DOI] [Google Scholar]
- 36.McKey, D. The distribution of secondary compounds within plants in Herbivores: their interaction with secondary plant metabolites (eds Rosenthal, G. A. & Janzen, D. H.) 55–133 (Academic Press, New York, 1979).
- 37.Wink, M. Compartmentation of secondary metabolites and xenobiotics in plant vacuoles in The Plant Vacuole: Advances in Botanical Research (eds Leigh, R. A., Sanders, D. & Callow, J. A.) 141–170 (Academic Press, London, 1997).
- 38.Philippi J, Schliephake E, Juergens HU, Jansen G, Ordon F. Feeding behavior of aphids on narrow-leafed lupin (Lupinus angustifolius) genotypes varying in the content of quinolizidine alkaloids. Entomol. Exp. Appl. 2015;156:37–51. doi: 10.1111/eea.12313. [DOI] [Google Scholar]
- 39.Golawska S. Deterrence and toxicity of plant saponins for the pea aphid Acyrthosiphon pisum Harris. J. Chem. Ecol. 2007;33:1598–1606. doi: 10.1007/s10886-007-9333-y. [DOI] [PubMed] [Google Scholar]
- 40.Golawska S, Lukasik I. Antifeedant activity of luteolin and genistein against the pea aphid. Acyrthosiphon pisum. J. Pest Sci. 2012;85:443–450. doi: 10.1007/s10340-012-0452-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Golawska S, Sprawka I, Lukasik I. Effect of saponins and apigenin mixtures on feeding behavior of the pea aphid, Acyrthosiphon pisum Harris. Biochem. Syst. Ecol. 2014;55:137–144. doi: 10.1016/j.bse.2014.03.010. [DOI] [Google Scholar]
- 42.Leszczynski B, Tjallingii WF, Dixon AFG, Swiderski R. Effect of methoxyphenols on grain aphid feeding behavior. Entomol. Exp. Appl. 1995;76:157–162. doi: 10.1111/j.1570-7458.1995.tb01957.x. [DOI] [Google Scholar]
- 43.Takemura M, Nishida R, Mori N, Kuwahara Y. Acylated flavonol glycosides as probing stimulants of a bean aphid, Megoura crassicauda, from Vicia angustifolia. Phytochemistry. 2002;61:135–140. doi: 10.1016/S0031-9422(02)00226-1. [DOI] [PubMed] [Google Scholar]
- 44.Lattanzio V, Lattanzio VM, Cardinali A. Role of phenolics in the resistance mechanisms of plants against fungal pathogens and insects. Phytochemistry: Adv. Res. 2006;661:23–67. [Google Scholar]
- 45.Ainsworth EA, Yendrek CR, Skoneczka JA, Long SP. Accelerating yield potential in soybean: potential targets for biotechnological improvement. Plant Cell Environ. 2012;35:38–52. doi: 10.1111/j.1365-3040.2011.02378.x. [DOI] [PubMed] [Google Scholar]
- 46.Bortier, K., Ceulemans, R. & de Temmerman, L. Effects of tropospheric ozone on woody plants in Environmental pollution and plant responses (eds Agrawal, S. B. & Agrawal, M.) 153–182 (CRC, Boca Raton, FL, 2000).
- 47.Li XM, Zhang LH, Ma LJ, Li YY. Elevated carbon dioxide and/or ozone concentrations induce hormonal changes in Pinus tabulaeformis. J. Chem Ecol. 2011;37:779–784. doi: 10.1007/s10886-011-9975-7. [DOI] [PubMed] [Google Scholar]
- 48.Mishra AK, Rai R, Agrawal S. Individual and interactive effects of elevated carbon dioxide and ozone on tropical wheat (Triticum aestivum L.) cultivars with special emphasis on ROS generation and activation of antioxidant defence system. Indian J Biochem. Bio. 2013;50:139–149. [PubMed] [Google Scholar]
- 49.Hoshika Y, Watanabe M, Inada N, Koike T. Growth and leaf gas exchange in three birch species exposed to elevated ozone and CO2 in summer. Water Air Soil Poll. 2012;223:5017–5025. doi: 10.1007/s11270-012-1253-y. [DOI] [Google Scholar]
- 50.Vogt T. Phenylpropanoid biosynthesis. Mol. Plant. 2010;3:2–20. doi: 10.1093/mp/ssp106. [DOI] [PubMed] [Google Scholar]
- 51.Douglas CJ. Phenylpropanoid metabolism and lignin biosynthesis: From weeds to trees. Trends Plant Sci. 1996;1:171–178. doi: 10.1016/1360-1385(96)10019-4. [DOI] [Google Scholar]
- 52.Zavala JA, Nabity PD, DeLucia EH. An emerging understanding of mechanisms govering insect herbivory under elevated CO2. Annu. Rev. Entomol. 2013;58:79–97. doi: 10.1146/annurev-ento-120811-153544. [DOI] [PubMed] [Google Scholar]
- 53.Gupta, R. et al. A multi-omics analysis of Glycine max leaves reveals alteration in flavonoid and isoflavonoid metabolism upon ethylene and abscisic acid treatment. Proteomics, 1700366 (2018). [DOI] [PubMed]
- 54.Ghasemzadeh A, Jaafar HZE, Rahmat A. Elevated carbon dioxide increases contents of flavonoids and phenolic compounds, and antioxidant activities in Malaysian young ginger (Zingiber officinale Roscoe.) varieties. Molecules. 2010;15:7907–7922. doi: 10.3390/molecules15117907. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.O’Neill BF, et al. Impact of elevated levels of atmospheric CO2 and herbivory on flavonoids of soybean (Glycine max Linnaeus) J. Chem. Ecol. 2010;36:35–45. doi: 10.1007/s10886-009-9727-0. [DOI] [PubMed] [Google Scholar]
- 56.Elger A, Lemoine DG, Fenner M, Hanley ME. Plant ontogeny and chemical defence: older seedlings are better defended. Oikos. 2009;118:767–773. doi: 10.1111/j.1600-0706.2009.17206.x. [DOI] [Google Scholar]
- 57.Rai R, Agrawal M. Assessment of competitive ability of two Indian wheat cultivars under ambient O3 at different developmental stages. Environ. Sci. Pollut. R. 2014;21:1039–1053. doi: 10.1007/s11356-013-1981-6. [DOI] [PubMed] [Google Scholar]
- 58.Barton KE, Koricheva J. The ontogeny of plant defense and herbivory: characterizing general patterns using meta-analysis. Am. Nat. 2010;175:481–493. doi: 10.1086/650722. [DOI] [PubMed] [Google Scholar]
- 59.Morgan P, Ainsworth E, Long S. How does elevated ozone impact soybean? A meta‐analysis of photosynthesis, growth and yield. Plant Cell Environ. 2003;26:1317–1328. doi: 10.1046/j.0016-8025.2003.01056.x. [DOI] [Google Scholar]
- 60.Kangasjärvi J, Talvinen J, Utriainen M, Karjalainen R. Plant defense systems induced by ozone. Plant Cell Environ. 1994;17:783–794. doi: 10.1111/j.1365-3040.1994.tb00173.x. [DOI] [Google Scholar]
- 61.Gillespie KM, et al. Greater antioxidant and respiratory metabolism in field-grown soybean exposed to elevated O3 under both ambient and elevated CO2. Plant Cell Environ. 2012;35:169–184. doi: 10.1111/j.1365-3040.2011.02427.x. [DOI] [PubMed] [Google Scholar]
- 62.Simmonds MSJ. Flavonoid-insect interactions: recent advances in our knowledge. Phytochemistry. 2003;64:21–30. doi: 10.1016/S0031-9422(03)00293-0. [DOI] [PubMed] [Google Scholar]
- 63.Meng F, Han Y, Teng W, Li Y, Li W. QTL underlying the resistance to soybean aphid (Aphis glycines Matsumura) through isoflavone-mediated antibiosis in soybean cultivar ‘Zhongdou 27′. Theor. Appl. Genet. 2011;123:1459–1465. doi: 10.1007/s00122-011-1680-y. [DOI] [PubMed] [Google Scholar]
- 64.Torto B, Hassanali A, Saxena KN, Nokoe S. Feeding responses of Chilo partellus (Swinhoe) (Lepidoptera, Pyralidae) larvae to sorghum plant phenolics and their analogs. J. Chem. Ecol. 1991;17:67–78. doi: 10.1007/BF00994422. [DOI] [PubMed] [Google Scholar]
- 65.Serratos A, et al. Factors contributing to resistance of exotic maize populations to maize weevil. Sitophilus zeamais. J. Chem. Ecol. 1987;13:751–762. doi: 10.1007/BF01020157. [DOI] [PubMed] [Google Scholar]
- 66.Chrzanowski G, et al. Effect of phenolic acids from black currant, sour cherry and walnut on grain aphid (Sitobion avenae F.) development. Crop Prot. 2012;35:71–77. doi: 10.1016/j.cropro.2012.01.005. [DOI] [Google Scholar]
- 67.Li Q, Eigenbrode SD, Stringham GR, Thiagarajah MR. Feeding and growth of Plutella xylostella and Spodoptera eridania on Brassica juncea with varying glucosinolate concentrations and myrosinase activities. J. Chem. Ecol. 2000;26:2401–2419. doi: 10.1023/A:1005535129399. [DOI] [Google Scholar]
- 68.Kliebenstein D, Pedersen D, Barker B, Mitchell-Olds T. Comparative analysis of quantitative trait loci controlling glucosinolates, myrosinase and insect resistance in Arabidapsis thaliana. Genetics. 2002;161:325–332. doi: 10.1093/genetics/161.1.325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Arany AM, et al. Glucosinolates and other metabolites in the leaves of Arabidopsis thaliana from natural populations and their effects on a generalist and a specialist herbivore. Chemoecology. 2008;18:65–71. doi: 10.1007/s00049-007-0394-8. [DOI] [Google Scholar]
- 70.Zavala JA, Mazza CA, Dillon FM, Chludil HD, Ballare CL. Soybean resistance to stink bugs (Nezara viridula and Piezodorus guildinii) increases with exposure to solar UV-B radiation and correlates with isoflavonoid content in pods under field conditions. Plant cell Environ. 2015;38:920–928. doi: 10.1111/pce.12368. [DOI] [PubMed] [Google Scholar]
- 71.Vieira SS, et al. Biological aspects of Bemisia tabaci biotype B and the chemical causes of resistance in soybean genotypes. Arthropod-Plant Inte. 2016;10:525–534. doi: 10.1007/s11829-016-9458-4. [DOI] [Google Scholar]
- 72.Guo H, et al. Elevated CO2 alters the feeding behaviour of the pea aphid by modifying the physical and chemical resistance of Medicago truncatula. Plant Cell Environ. 2014;37:2158–2168. doi: 10.1111/pce.12306. [DOI] [PubMed] [Google Scholar]
- 73.Guo H, et al. Up-regulation of MPK4 increases the feeding efficiency of the green peach aphid under elevated CO2 in Nicotiana attenuata. J. Exp. Bot. 2017;68:5923–5935. doi: 10.1093/jxb/erx394. [DOI] [PubMed] [Google Scholar]
- 74.Sun Y, et al. Plant stomatal closure improves aphid feeding under elevated CO2. Global Change Biol. 2015;21:2739–2748. doi: 10.1111/gcb.12858. [DOI] [PubMed] [Google Scholar]
- 75.Newman J, Gibson D, Parsons A, Thornley J. How predictable are aphid population responses to elevated CO2? J. Anim. Ecol. 2003;72:556–566. doi: 10.1046/j.1365-2656.2003.00725.x. [DOI] [PubMed] [Google Scholar]
- 76.Tosh CR, Powell G, Holmes ND, Hardie J. Reproductive response of generalist and specialist aphid morphs with the same genotype to plant secondary compounds and amino acids. J. Insect Physiol. 2003;49:1173–1182. doi: 10.1016/j.jinsphys.2003.08.014. [DOI] [PubMed] [Google Scholar]
- 77.Holopainen JK, Kössi S. Variable growth and reproduction response of the spruce shoot aphid, Cinara pilicornis, to increasing ozone concentrations. Entomol. Exp. Appl. 1998;87:109–113. doi: 10.1046/j.1570-7458.1998.00311.x. [DOI] [Google Scholar]
- 78.Couture JJ, Meehan TD, Lindroth RL. Atmospheric change alters foliar quality of host trees and performance of two outbreak insect species. Oecologia. 2012;168:863–876. doi: 10.1007/s00442-011-2139-1. [DOI] [PubMed] [Google Scholar]
- 79.Dermody O, O’Neill BF, Zangerl AR, Berenbaum MR, DeLucia EH. Effects of elevated CO2 and O3 on leaf damage and insect abundance in a soybean agroecosystem. Arthropod-Plant Inte. 2008;2:125–135. doi: 10.1007/s11829-008-9045-4. [DOI] [Google Scholar]
- 80.Ng JC, Perry KL. Transmission of plant viruses by aphid vectors. Mol. Plant Pathol. 2004;5:505–511. doi: 10.1111/j.1364-3703.2004.00240.x. [DOI] [PubMed] [Google Scholar]
- 81.Fereres A, Moreno A. Behavioural aspects influencing plant virus transmission by homopteran insects. Virus Res. 2009;141:158–168. doi: 10.1016/j.virusres.2008.10.020. [DOI] [PubMed] [Google Scholar]
- 82.Tjallingii W, Hogen-Esch T. Fine structure of aphid stylet routes in plant tissues in correlation with EPG signals. Physiol. Entomol. 1993;18:317–328. doi: 10.1111/j.1365-3032.1993.tb00604.x. [DOI] [Google Scholar]
- 83.Oleszek W, Stochmal A. Triterpene saponins and flavonoids in the seeds of Trifolium species. Phytochemistry. 2002;61:165–170. doi: 10.1016/S0031-9422(02)00230-3. [DOI] [PubMed] [Google Scholar]
- 84.Nour V, Trandafir I, Cosmulescu S. HPLC determination of phenolic acids, flavonoids and juglone in walnut leaves. J. Chromatogr. Sci. 2013;51:883–890. doi: 10.1093/chromsci/bms180. [DOI] [PubMed] [Google Scholar]
- 85.Lattanzio V, Arpaia S, Cardinali A, Di Venere D, Linsalata V. Role of endogenous flavonoids in resistance mechanism of Vigna to aphids. J. Agr. Food Chem. 2000;48:5316–5320. doi: 10.1021/jf000229y. [DOI] [PubMed] [Google Scholar]