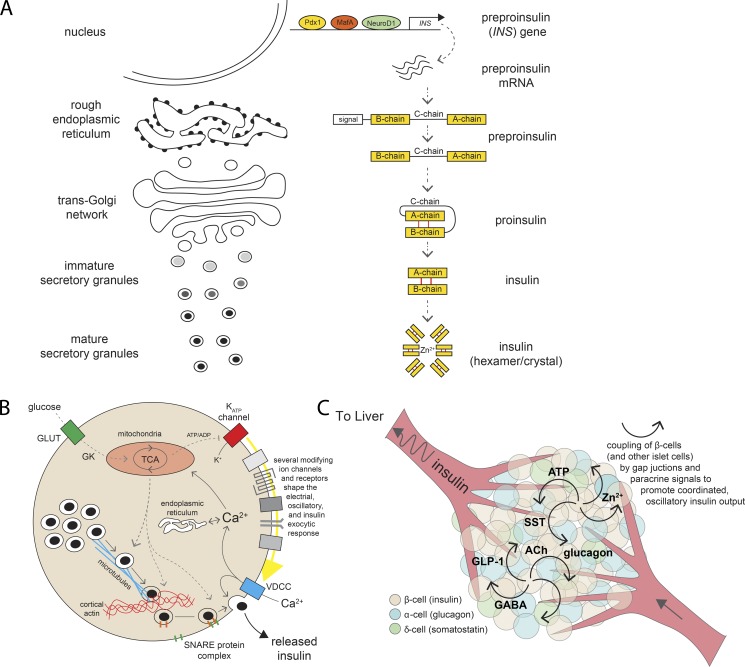
Figure 2.
Insulin biosynthesis and secretion. (A) Insulin maturation along the granule secretory pathway. Preproinsulin mRNA is transcribed from the INS gene and translated to preproinsulin peptide. As this transits through the RER and TGN, the prepropeptide is processed to its mature form and ultimately stored as hexameric insulin/Zn2+ crystals within mature secretory granules. (B) Glucose sensing and metabolic signals leading to insulin granule secretion. The release of insulin via exocytosis of secretory granules from pancreatic β-cells is controlled by a series of metabolic and electrical signals arising as a result of glucose entry through GLUTs, phosphorylation by GK, and entry into the TCA cycle. The closure of ATP-dependent K+ (KATP) channels triggers electrical events that culminate in Ca2+ entry through voltage-dependent Ca2+ channels (VDCCs), which triggers exocytosis mediated by SNARE complex proteins. The overall secretory response is modulated by numerous receptors, channels, intracellular Ca2+ stores, metabolic signals, and cytoskeletal elements. (C) Islet communication for coordinated pulsatile insulin secretion. Within an islet, β-cells communicate with each other and with glucagon-producing α-cells and somatostatin (SST)–producing δ-cells to coordinate their activity. Many putative intraislet messengers have been implicated, including ATP, Zn2+, γ-aminobutyric acid (GABA), glucagon-like peptide-1 (GLP-1), acetylcholine (ACh), and others. These, along with electrical coupling via gap junctions, are likely important for the physiological coordination of pulsatile insulin secretion.