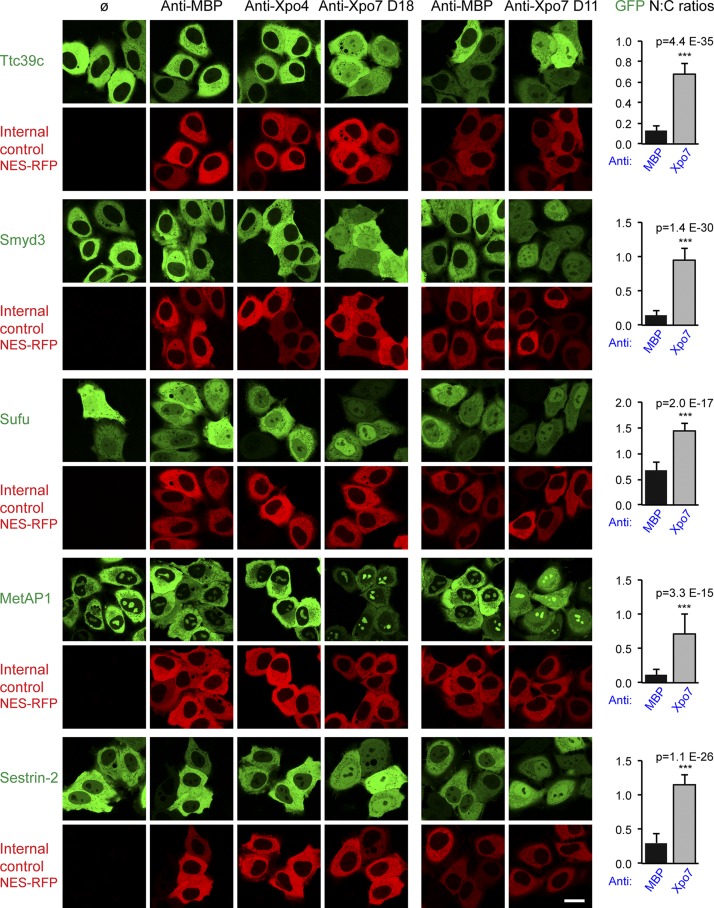
Figure 4.
Validation of export cargo candidates. GFP-fused candidate proteins from mouse were transiently expressed in HeLa cells, and their subcellular localization was recorded in live cells by confocal fluorescence microscopy. The effect of the nanobodies recognizing MBP, Xpo4, or Xpo7 (D18) was monitored by cotransfection with a vector encoding NES-RFP to stain the cytoplasmic compartment. In a separate experiment, anti-Xpo7 nanobody D11 was tested. Bar, 20 µm. The predominant cytoplasmic localization of Ttc39c (Q8VE09), Smyd3 (Q9CWR2), MetAP1 (Q8BP48), and Sestrin-2 (P58043) was disrupted only by anti-Xpo7 nanobodies, which led to nuclear accumulation. This suggests that these proteins can leak into nuclei and that they are kept cytoplasmic at steady state by Xpo7-dependent export. A milder disruption was observed for Sufu (Q9Z0P7), where block of Xpo7 increased the relative nuclear GFP signal. UniProt entry names are listed in parentheses. On the right, mean ratios of nuclear/cytoplasmic (N:C) GFP concentrations are plotted, with anti-MBP and anti-Xpo7 nanobody results averaged (n = 20–40). Error bars represent SD. Statistical significance (***, p < 10−6) was assessed using an unpaired two-sided t test. Means ± SD of each sample are listed in Table S1.