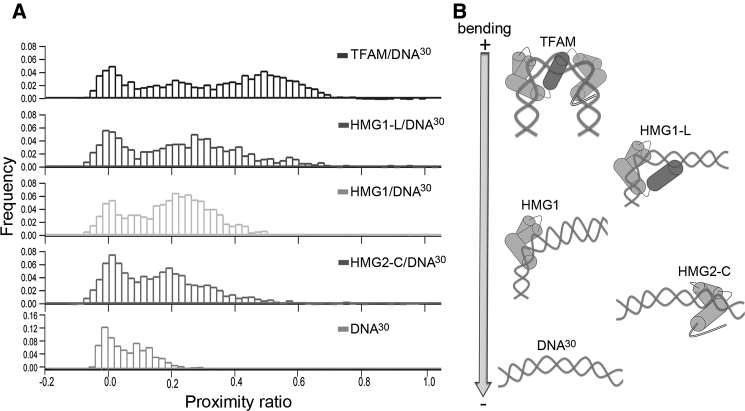
Figure 3.
The individual protein domains orchestrate stepwise DNA bending. (A) Representative smFRET histograms for TFAM and TFAM domains in complex with DNA30 are shown. The complex with full-length TFAM is shown at the top, followed by HMG1-L, HMG1, HMG2-Cter, and free DNA (bottom). (B) A model of bending is represented aligned with each histogram and ordered from top (sharpest) to bottom (lowest) according to the extent of DNA bending. The TFAM/DNA model is based on the U-turn structure, whereas the complexes with domains are hypothetical representations based on the proximity ratio distributions. The DNA is represented as prebent, in accordance with our MD results. From bottom to top, the free DNA and HMG2 complex show similar bending due to the marginal effect of HMG2. Above, HMG1 introduces the first kink, which is enhanced by the linker. At the top, full-length TFAM induces a U-turn, suggesting cooperativity, because HMG2 kinks the DNA only in the presence of the N-terminal protein fragment.