Figure 5.
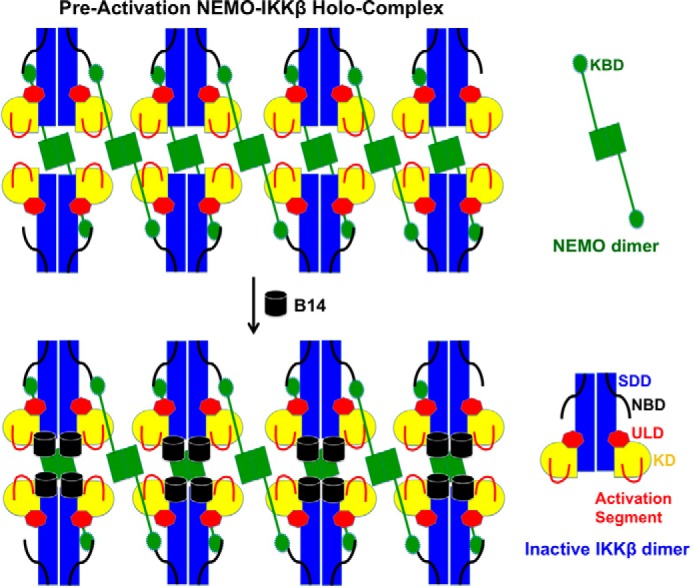
A model for B14-mediated inhibition of IKKβ activation. The KBD of NEMO interacts with the NBD of IKKβ, which cross-links IKKβ to a large oligomer. Upon activation by upstream signaling events or by high IKK concentration, the activation segments of the neighboring KDs can contact each other for trans autophosphorylation. Binding of B14 to the junction of KD and SDD of IKKβ causes a steric hindrance that impedes the optimal contact between KDs in the IKK complex, which, in turn, blocks the insertion of the activation segment of one KD to the active site of another for trans autophosphorylation and activation.
