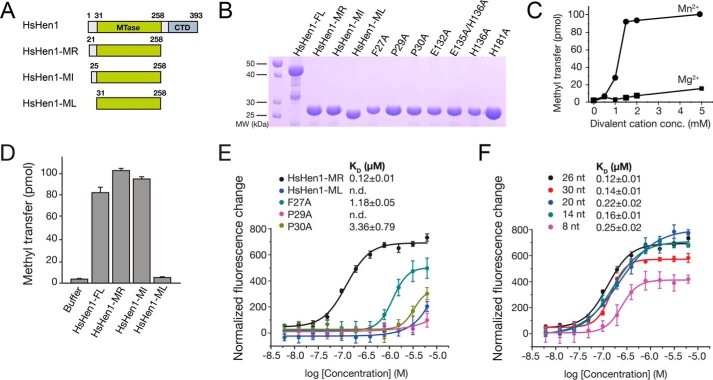
Figure 5.
FXPP motif that is specific in eukaryotic Hen1 is important for RNA binding. A, schematic representation of human Hen1 and three truncated mutants. B, purified WT or mutant HsHen1 used in the methyltransferase assays or FP assays were detected by 15% SDS-PAGE and visualized by Coomassie Blue staining. Purified HsHen1-FL (residues 1–393), HsHen1-MR (residues 21–258), HsHen1-MI (residues 25–258), HsHen1-ML (residues 31–258), and HsHen1-MR mutants (F27A, P29A, P30A, E132A, E135A/E136A, H136A, and H181A) are indicated from left to right. C, in vitro methyltransferase assays showed that HsHen1-MR prefers manganese over magnesium. 0, 0.5, 1, 1.5, 2, or 5 mm either of MnCl2 or MgCl2 as specified were added and incubated in 37 °C for 45 min. D, in vitro methyltransferase assays of WT and mutant HsHen1. The averages of three independent experiments with S.D. values are shown. E and F, binding affinities between HsHen1 proteins and FAM-labeled RNA oligos. The fluorescence intensity changes under polarized illumination were plotted. Error bars indicate standard deviation of the mean (n = 3). E, fluorescence polarization assay showed affinities between HsHen1-ML, WT, or mutant HsHen1-MR and a 26-nt fluorescently-labeled RNA. Color scheme of different HsHen1 proteins is indicated. F, fluorescence polarization assay showed affinities between HsHen1-MR and different lengths of FAM-labeled RNA oligos. Color scheme of different length of FAM-labeled RNA oligos is indicated.