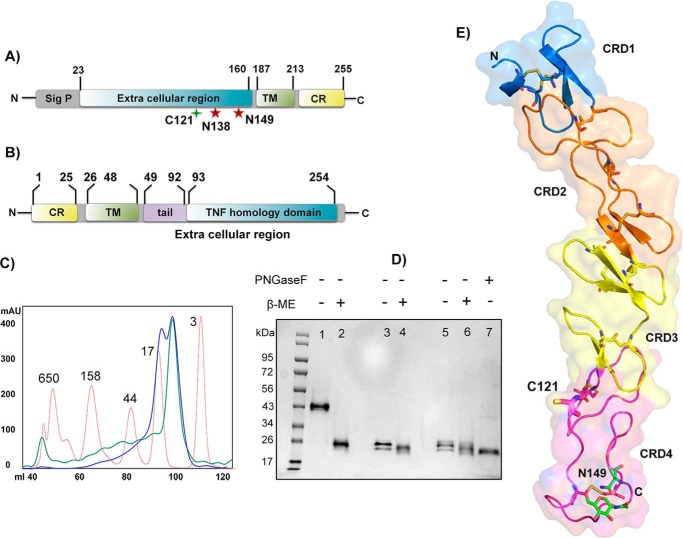
Figure 1.
Protein constructs, expression, and h4-1BB structure. A and B, domain architecture of h4-1BB (A) and h4-1BBL (B). Sig p, signal peptide; TM, transmembrane region; CR, cytoplasmic region. The unpaired cysteine 121 and two potential N-linked glycosylation sites of h4-1BB are indicated. C, size exclusion profile of purified wildtype (WT) (blue line) and C121S mutant (green line) of h4-1BB with reference to molecular mass marker proteins (red line) in kDa. D, SDS-PAGE analysis of purified WT and C121S mutant of h4-1BB under nonreducing (−β-ME (lanes 1, 3, and 5) and reducing conditions (+β-ME (lanes 2, 4, and 6). Lanes 1 and 2 contain dimeric WT 4-1BB (blue high Mr peak from C) and lanes 3 and 4 contain monomeric WT 4-1BB (blue low Mr peak from C). Lanes 5 and 6 contain the C121S mutant 4-1BB (green low Mr peak from C). Lane 7 represents deglycosylated (PNGase (peptide N-glycosidase F) treated) WT 4-1BB. E, crystal structure of h4-1BB (in the 4-1BB–4-1BBL complex) colored by the four cysteine-rich domains as cartoon overlaid onto transparent molecular surface representation. Unpaired Cys121 and disulfide bridges are shown as sticks in respective colors of CRDs. The N-linked glycosylation site and the N-glycans are represented as sticks with carbon atoms in green, oxygen in red, and nitrogen atoms in blue.