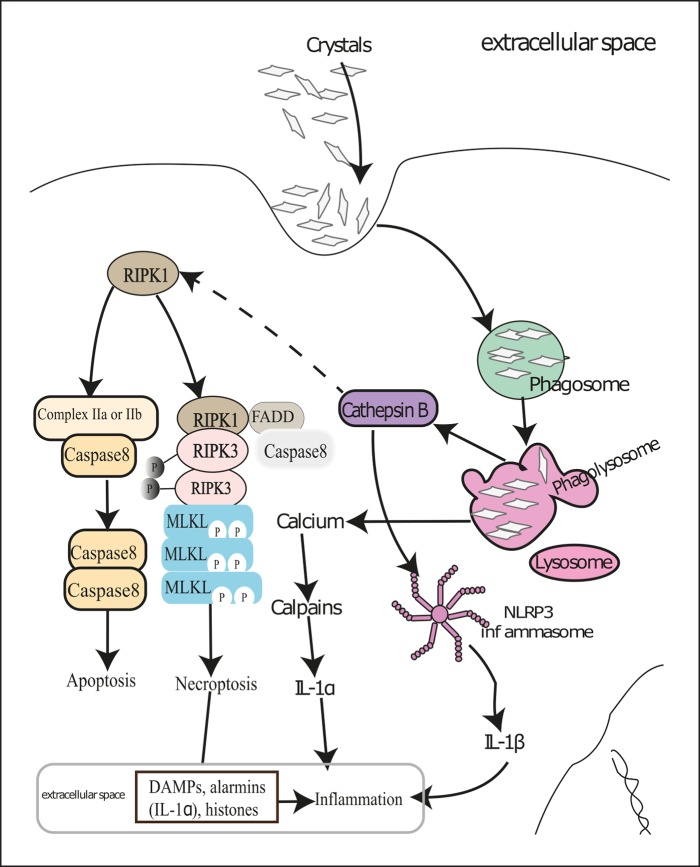
Fig. 2.
Molecular mechanisms of crystal-induced necroinflammation. Renal cells try to digest crystals deposited in kidneys in phagolysosomes after engulfing them by phagocytosis. This results in Ca2+ release in the cytosol and subsequent activation of calpains leading to IL-1α release, which sets up inflammation. However, bigger and indigestible crystals induce lysosomal destabilization and thereby release cathepsin B in the cytosol. Cathepsin B and reactive oxygen species activate the NACHT, LRR, and PYD domains containing protein 3 (NLRP3) inflammasome and cause secretion of mature IL-1β. Cathepsin B also cleaves RIPK1, which leads to either caspase-mediated apoptosis or RIPK3- and MLKL-mediated cellular necroptosis in the absence of caspase 8. Dying renal cells release various DAMPs and alarmins that contribute to renal inflammation. Certain pro-inflammatory cytokines can also induce regulated cell death in neighboring live cells and thus set up an auto-amplification loop of necroinflammation. DAMPs, danger-associated molecular patterns; MLKL, mixed lineage kinase domain-like; RIPK1, receptor-interacting serine/threonine-protein kinase 1; RIPK3, receptor-interacting serine/threonine-protein kinase 3.