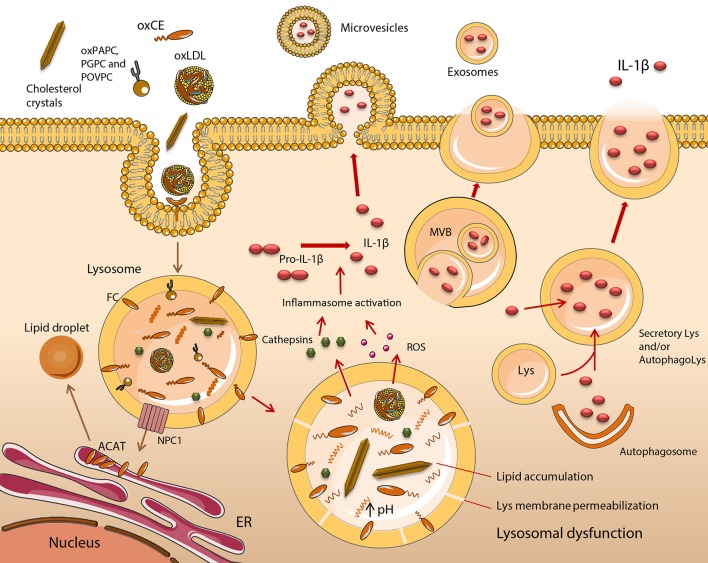
Figure 1.
Functional impairment of lipid-engorged lysosomes stimulates inflammasome assembly and IL-1β release. The uptake of lipid and lipoprotein molecules occurs by a variety of scavenger receptor (SR), and Toll-like receptor (TLR)-mediated mechanisms such as phagocytosis or macropinocytosis. In early atherogenesis, monocyte (Mo)-derived macrophages (MOs) retain lipids and lipoproteins, which become degraded in lysosomes, with excess free cholesterol (FC) trafficked to the endoplasmic reticulum (ER). Cholesteryl esters (CE) are hydrolyzed in lysosomes by lysosomal acid lipase (LAL) to FC and fatty acids. FC is trafficked to the ER via Niemann Pick C1 (NPC1), where it becomes esterified by acyl CoA:cholesterol acyltransferase (ACAT), forming CE, which are packaged into cytoplasmic lipid droplets. This is a distinctive feature of foam cell formation. It is induced upon MO cholesterol loading, with lysosomal hydrolysis vital for the mobilization of lipid droplet-associated cholesterol for reverse cholesterol transport. This mechanism of sterol clearance is initially effective, however, when fatty streak lesions degenerate into unstable plaques, a substantial accumulation of CEs, their oxidized derivatives (oxCEs), and FC occurs in lysosomes due to inadequate hydrolysis and clearance. Lysosome dysfunction in excess lipid-loaded MOs is irreversible and is characterized by an inhibition of LAL and cathepsin activity, due to permanent alterations in lysosome function, resulting in the accumulation of cargo. Dysfunctional lysosomes become ruptured, releasing cathepsins, reactive oxygen species (ROS) and other molecules into the cytoplasm. These activate the NLRP3 inflammasome, leading to the maturation and release of IL-1β. Five potential mechanisms of IL-1β release have been described including the exocytosis of secretory lysosomes, exosomes and microvesicle shedding. Lys, lysosome; MVB, multivesicular body.