We are very thankful to Crassini et al. for evaluating the impact of infections on overall survival (OS) in their long-term follow-up cohort as a commentary to our paper on predicting infection in CLL.1 They report a significant association between infection during the first year of observation and OS. In the last couple of years, our groups and others have examined the immune dysfunction, both in general but particularly hypogammaglobulinemia, in patients with CLL. Both groups have demonstrated poor survival among CLL patients with hypogammaglobulinemia. Now, Crassini et al. demonstrate that identifying immune dysfunction in terms of early infection is predictive of poor OS.
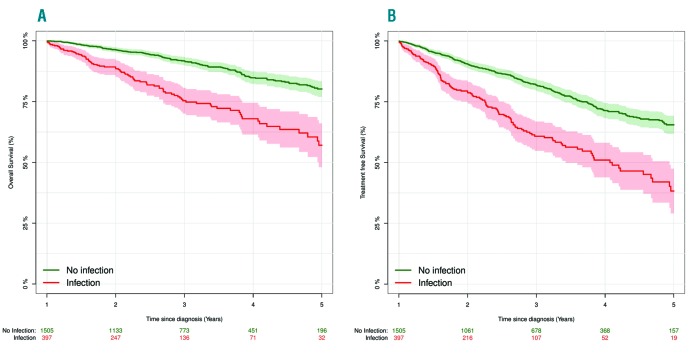
Within our nationwide cohort of patients diagnosed with CLL in Denmark since 2008, we re-analyzed OS and treatment free survival (TFS) based on whether patients have had an infection during the first year after diagnosis or not.2 All patients alive and treatment-naïve one year after CLL diagnosis were included for this analysis. As demonstrated below, the association (P<0.001) between infection, OS and TFS, as reported by Crassini et al., could be validated in this nationwide cohort (Figure 1 A and B).
Figure 1.
Kaplan-Meier curves for A) overall survival and B) treatment free survival. (A) Kaplan-Meier curve from one year after diagnosis until death or end of follow up for all included patients grouped by patients having an infection during the first year (red). (B) Kaplan-Meier curve from one year after diagnosis until death, treatment or end of follow up for all included patients grouped by patients having an infection during the first year (red).
When discussing immune dysfunction in CLL in the era of targeted therapies, three important questions warrant further assessment:
Are we able to identify patients with immune dysfunction prior to significant morbidity or mortality due to infections? The data presented in the original paper and in the work by Crassini et al. contribute further to answering this question; at EHA 2018, we present the first machine learning based approach to identifying patients at high risk of infection at diagnosis of CLL.
Are we able to change the microenvironmental interaction and thus the immune dysfunction in CLL by targeted agents? For ibrutinib, Sun et al. showed an increase in IgA after 6 months of treatment and several publications have demonstrated changes in microenvironmental interaction upon clinical use of ibrutinib.3–6
Most importantly, will we be able to change the natural history of CLL and thereby decrease morbidity and mortality due to infections and immune dysfunction in CLL? To address this question, we are currently developing a randomized clinical trial of pre-emptive treatment in patients newly diagnosed with CLL, who are at high risk of infection but do not meet the IWCLL indication for treatment.
Supplementary Material
Footnotes
Information on authorship, contributions, and financial & other disclosures was provided by the authors and is available with the online version of this article at www.haematologica.org.
References
- 1.Andersen MA, Eriksen CT, Brieghel C, et al. Incidence and predictors of infection among patients prior to treatment of chronic lymphocytic leukemia: a Danish nationwide cohort study. Haematologica. 2018. March 8 [Epub ahead of print] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.da Cunha-Bang C, Geisler CH, Enggaard L, et al. The Danish National Chronic Lymphocytic Leukemia Registry. Clin Epidemiol. 2016;8:561–565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Sun C, Tian X, Lee YS, et al. Partial reconstitution of humoral immunity and fewer infections in patients with chronic lymphocytic leukemia treated with ibrutinib. Blood. 2015;19(19):2213–2219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Burger JA. Nurture versus Nature: The microenvironment in chronic lymphocytic leukemia. Hematology Am Soc Hematol Educ Program. 2011;2011:96–103. [DOI] [PubMed] [Google Scholar]
- 5.Woyach JA, Bojnik E, Ruppert AS, et al. Bruton’s tyrosine kinase (BTK) function is important to the development and expansion of chronic lymphocytic leukemia (CLL). Blood. 2014;123(8):1207–1213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Niemann CU, Herman SEM, Maric I, et al. Disruption of in vivo chronic lymphocytic leukemia tumor–microenvironment interactions by ibrutinib – findings from an investigator-initiated phase II study. Clin Cancer Res. 2016;22(7):1572–1582. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.