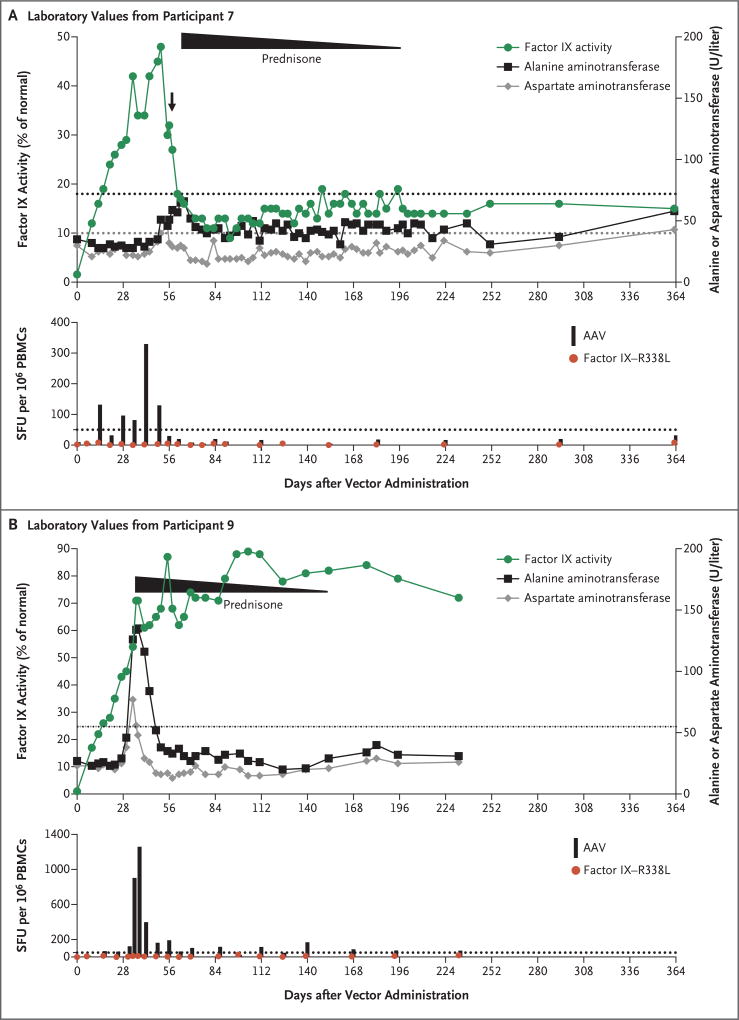
Figure 2. Laboratory Values in Two Participants Who Had an Immune Response to the Adeno-Associated Viral (AAV) Vector.
Evidence of an immune response was determined by monitoring each participant’s factor IX activity and alanine aminotransferase and aspartate aminotransferase levels and by evaluating the results of the interferon-γ enzyme-linked immunosorbent spot (ELISPOT) assay regarding the reaction of the participant’s peripheral-blood mono nuclear cells (PBMCs) to the AAV capsid peptides and the transgene product (factor IX–R338L), as compared with a media control. The dotted lines in the top graph in each panel indicate the upper limit of the normal range for the alanine aminotransferase (black) and aspartate aminotransferase (gray) levels in each participant, according to the local laboratory. The arrow after day 56 in Panel A (showing values for Participant 7) indicates the receipt of a single dose of prednisone. The duration of a full course prednisone treatment (started a few days later) is shown, with the slope indicating tapering. The levels of alanine aminotransferase and aspartate aminotransferase returned to baseline levels in these two participants after prednisone initiation, and transgene expression was maintained in the two participants after prednisone was tapered and stopped. Results of the ELISPOT assay are shown as the number of spot-forming units (SFU) per 1 million PBMCs; values that are more than 50 SFU or that are above the media control (dotted line) by a factor of three are considered to be positive.