Abstract
There are established effects of self- and reward-biases even on simple perceptual matching tasks [Sui, J., He, X., & Humphreys, G. W. (2012). Perceptual effects of social salience: Evidence from self-prioritization effects on perceptual matching. Journal of Experimental Psychology, Human Perception and Performance, 38, 1105–1117]; however we know little about whether these biases can be modulated by particular interventions, and whether the biases then change in the same way. Here we assessed how the biases alter under conditions designed to induce negative mood. We had participants read a list of self-related negative or neutral mood statements [Velten, E. (1968). A laboratory task for induction of mood states. Behavior Research and Therapy, 6, 473–482] and also listen for 10 min to a passage of negative or neutral music, prior to carrying out perceptual matching with shapes associated to personal labels (self or stranger) or reward (£12 or £1). Responses to the self- and high-reward-associated shapes were selectively slower and less sensitive (d′) following the negative mood induction procedures, and the decrease in mood correlated with decreases in the reaction time bias across “high saliency” (self and high-reward) stimuli. We suggest that negative mood may decrease self- and reward-biases through reducing attention to salient external stimuli.
Keywords: Self-bias, Reward-bias, Negative mood, Associative learning
Recent work has established that both perception and memory can be modulated by a variety of biases reflecting (for example) whether a stimulus is personally linked to a participant and whether a stimulus has an associated reward value. For instance, Sui, He, and Humphreys (2012) had participants associate simple geometric shapes either with personal labels (circle–you, square–friend, triangle–stranger) or with different reward values (e. g., circle–£9, square–£3, triangle–£1). Participants then saw either the original pairings (as above) or shapes and labels re-paired (e.g., circle–friend, square–you, or circle–£3, square–£9). They found highly robust biases in perceptual matching for self-related stimuli (e.g., reaction times, RTs, for circle–you < RTs for square–friend) and for high-reward stimuli (RTs for circle–£9 < RTs for square–£3). In addition, responses to self- and high-reward stimuli were less affected by degrading stimulus contrast than responses to stimuli linked to other people and low rewards. Thus both self-bias and reward-bias effects interact with manipulations of the perceptual quality of the shapes, consistent with effects of self-reference and reward on perceptual processing.
The effects on perceptual matching also link to enhanced binding of information. Using a redundancy gain paradigm, researchers have reported that performance with two items is better than that with single items (the redundancy gain; e.g., Miller, 1982). These redundancy gains are larger when the items group to form a single object (Mordkoff & Danek, 2011), and they are larger for self-related than for other-related stimuli (Sui, Yankouskaya, & Humphreys, 2015) In addition, responses to redundant self stimuli violated the independent processing of two items (cf. Miller, 1982), and they were processed with “super-capacity” (Townsend & Eidels, 2011). The data indicate that participants responded to redundant self-related shapes as a perceptual whole, consistent with self-reference enhancing perceptual integration.
There is evidence for improved integration processes in memory induced by self-reference (Cunningham, Turk, Macdonald, & Macrae, 2008; Wang, Humphreys, & Sui, 2016). For example, participants typically show better memory for items subjected to self-ownership decisions than for items assigned to ownership by others. Furthermore, for remembered items, participants are more accurate at judging that the stimuli have been categorized in relation to the self than that they have been categorized in relation to other people (i.e., on source memory tasks; Cunningham, Brady-Van den Bos, & Turk, 2011). This enhancement of source memory is consistent with the better integration of elements to their memorial context (see Sui & Humphreys, 2015, for an overview).
These results converge with other findings indicating that self- and reward-associations increase the attentional saliency of stimuli. For example, Sui, Liu, Mevorach, and Humphreys (2013) showed that associating a shape with the self altered responses to the shape when it was presented within a hierarchical form (a large shape made up from smaller shapes) in the same way as altering the perceptual saliency of the shape by increasing its contrast or blurring. Also overlapping brain regions were recruited when self- and when perceptually salient distractors had to be ignored. Anderson, Laurent, and Yantis (2011) had stimuli consistently associated with high and low reward values and found that distractors associated with high reward were subsequently difficult to ignore even when irrelevant to the task. Self- and reward-related stimuli may each capture attention and demand specific neural processes to then be ignored.
The question arises then whether these biases on perception, attention, and memory can be modulated under particular conditions. There is much work with both humans and animals indicating that emotional states affect cognitive performance (Padmala & Pessoa, 2014; Soto et al., 2009), including effects on reward and self-related information processing. For example, low mood has been shown to increase self-focus (e.g., Grimm et al., 2009; Pyszczynksi, Hamilton, Herring, & Greenberg, 1989; Salovey, 1992; Wood, Saltzberg, & Goldsamt, 1990) and to reduce attention/motivational responses to reward (Blood & Zatorre, 2001; Chiew & Braver, 2011; Hervás & Vázquez, 2013), particularly in depression (Eshel & Roiser, 2010; Henriques & Davidson, 2000). Broadly speaking, the change from neutral to low mood can be interpreted as a general shift away from exteroceptive value judgment towards a more internal focus on homeostatic interactions, or the resting state of the value input system (Paulus, 2007). Thus, the relationship between exteroceptive reward valuation and the self-concept may be affected by low mood (Northoff & Hayes, 2011). If low mood reduces attention to reward then we may predict that reward-based biases may decrease under conditions that induce negative mood (e.g., Grimm et al., 2009). The predictions related to self-bias are less clear, however. If low mood produces a focus of attention to internal states, then it is possible that self-bias effects could increase under low mood conditions, if participants become more focused on self-related stimuli than on stimuli related to other people. On the other hand, it can be argued that self-biases in perceptual matching do not depend on internal attention to the self but rather on attention to self-associated external stimuli. For example, Sui, Rotshtein, and Humphreys (2013) examined the neural basis of self-bias effects in perceptual matching. They found that self-related stimuli enhanced neural activity (compared with stimuli associated to other people) in the ventral–medial pre-frontal cortex (vmPFC) and the left posterior, superior temporal sulcus (LpSTS). The vmPFC has been linked to self processing in a variety of brain imaging studies (Decety & Sommerville, 2003; Northoff & Bermpohl, 2004; Uddin, Lacoboni, Lange, & Keenan, 2007) while the LpSTS has been related to attention to the external stimulus environment (Samson, Apperly, Chiavarino, & Humphreys, 2004). Sui, Rotshtein, et al. (2013) further showed that greater self-biases were associated with increased functional connectivity between the vmPFC and the LpSTS, suggesting that the biases arise from self-representations (in the vmPFC) priming attentional responses (in the LpSTS) to self-related external stimuli. If this is indeed the case, and if low mood reduces attention to external stimuli, then we might expect that inducing a low mood in participants would decrease self-biases in perceptual matching. This was tested here.
The current study also has the potential to prise apart the relations between self- and reward-biases in perception. As noted, Sui et al. (2012) found qualitatively similar effects of self- and reward-bias on perceptual matching. However, this does not mean that self- and reward-biases reflect the same underlying mechanisms, but merely that perceptual processing is modulated by both factors. Indeed, in their study of redundancy gains Sui et al. (2015) failed to find evidence for super-capacity for redundant high-reward stimuli, contrasting with the evidence with redundant self-related stimuli. This suggests that integration processes at a perceptual level may be less affected by reward than by self-association. By testing the effects of music intervention here on both self- and reward-biases, we evaluate whether the two biases are affected in the same way by a common intervention, and whether the same aspects of the intervention affect performance. In addition, we can assess whether changes in self-bias and changes in reward-bias, under the music intervention, are correlated across the participants. If there are common processes mediating the two bias effects, then the effects of mood change in one case (e.g., for self-bias) should follow the pattern in the other (e.g., for reward-bias).
To test the above hypotheses, we had participants perform self- and reward-related tasks after music intervention in two sessions (one session with negative music, the other with non-negative music as a baseline). In each session, a 10-min period in which participants listened to music was followed immediately by two perceptual matching tasks—one in which shapes were associated with personal labels and one in which the shapes were associated with labels for different reward values (Sui et al., 2012). At the end of each session, participants were asked to rate their mood state using a continuous scale (negative to positive). We measured the effect of music intervention on mood state as well as on the self- and reward-biases in the matching task. In addition, we assessed the relations between the valence of the negative mood and changes in self- and reward-bias. Does negative mood enhance or reduce the two biases in perceptual matching? Also, are the effects of mood induction on self- and reward-bias linked?
Experimental Study
Method
Participants
Twenty-three undergraduates from Oxford University participated in the experiment (13 males, ages 18–22 years, mean age = 19.17 ± 1.11 years). All participants were right-handed and had normal or corrected-to-normal vision. Informed consent was obtained from all individuals prior to the experiment according to procedures approved by the ethics committee of the Medical Sciences Division of Oxford University. Three participants who did not show a self-advantage effect in the neutral condition were excluded from further analyses. Three out of the remaining 20 participants also did not rate the mood statements according to the instruction.
Stimuli and tasks
Eight geometric shapes (triangle, circle, square, hexagon, pentagon, octagon, diamond, and ellipse) were assigned to labels representing two people (the participant and a stranger) or two reward values (£12–high reward and £1–low reward) in two sessions (neutral music intervention and negative music intervention), respectively. The assignment of the shapes to the different people and reward values, across the two music conditions, was counterbalanced across participants and sessions. For example, participants were told that “the circle represents a stranger, and the ellipse represents you” in the personal associative task, and “the hexagon represents £1, and the square represents £12” in the reward associative task (Sui et al., 2012). After the instruction to associate the label and the shape, participants immediately underwent a shape–label matching task to judge whether the shape–label pair presented on each trial matched or mismatched. A shape (covering 3.5° × 3.5° of visual angle) was displayed above a white central fixation cross (0.8° × 0.8° visual angle). One of two labels (“you” or “stranger” or “£12 or £1”, depending on the session) covering 1.76°/2.52° × 1.76° of visual angle was displayed below the fixation cross. Participants were also told that there were extra rewards linked to the high- and low-associated shapes. For this session, participants gained an additional maximal reward across all trials of £12 for correct responses to high-reward-associated shapes, and an additional maximum of £1 for low-reward-associated shapes. All the stimuli were displayed on a grey background. E-prime software (Version 2.0) was used to present the stimuli and to record responses. The experiment was run on a PC with a 22″ monitor (1920 × 1080 pixels) at 60 Hz.
Mood induction procedure
In accordance with the requirements of a local ethics committee, we combined the Velten mood induction procedure (Velten, 1968) and a music mood induction procedure, both of which have been demonstrated to be effective in inducing negative mood (Clark, 1983; Pignatiello, Camp, & Rasar, 1986; Westermann, Spies, Stahl, & Hesse, 1996). In the negative mood induction, participants were presented with the negative list of Velten statements and were played a looped recording of Chopin’s Prelude No. 6 in B Minor, performed by Maurizio Pollini on desktop speakers. For the neutral mood condition, participants were presented with the neutral list of Velten statements and a looped recording of Ballade, Op. 19, by Fauré, performed by David Korevaar. Each music piece took about 10 min.
Two sessions (neutral and negative music interventions) took place over two consecutive days. Half the participants carried out the session with the neutral music intervention on the first day and then a session with negative music intervention on the second day. The order of these two sessions was reversed for the other participants. In each session, the order of personal and reward tasks was counterbalanced across participants. At the end of each session, participants had to rate their mood state using a 7-point continuous scale (1 = very negative, 4 = neither positive nor negative, 7 = very positive). The scores were used to calculate the effect of music intervention on mood state.
Procedure
After the mood induction session (see above), participants underwent associative matching where they were instructed to link two shapes with the self or a stranger in the personal association task, and two shapes with £12 or £1 in the reward task, respectively. Participants were told that they would be rewarded extra bonus at the end of each session based on and proportional to their correct responses to shapes associated with the high and low reward values. There were no images of stimuli displayed during the instruction stage.1 After the instruction, participants had to judge whether a simultaneously presented shape and label pair matched. Each trial started a central fixation cross for 500 ms, followed by a shape–label pair at the centre of the screen for 100 ms. Half the pairings of the shape and label conformed to the instruction and were responded to as match trials; on the remaining trials the shapes and labels were re-paired to form mismatch trials. For mismatch trials, a shape was paired with the other labels (e.g., self shape with stranger label in the personal task, or £12-associated shape with £1 label in the reward task). The next frame was a 1000-ms blank field. Participants were encouraged to make a response as quickly and accurately as possible within this 1000-ms interval. A feedback message (correct, incorrect, or too slow!) was then given in the centre of the screen for 500 ms. Participants were also informed of their overall accuracy at the end of each block. There were four blocks of 60 trials following 10 practice trials. Thus there were 60 trials for each match and mismatch condition in each task.
Data analysis
First we examined whether the mood induction procedure was effective by comparing the change in reported mood scores in the neutral and negative music intervention sessions. Second, we assessed the effect of negative mood induction on self- and reward-biases. There was no trade-off between RTs and accuracy in either the self or the reward tasks, for any conditions. The accuracy results were assessed using d′, computed from performance on match trials contrasted against mismatch trials with the same shape (see Sui et al., 2012). For each analysis (RTs, d′) we (a) first measured the effect of negative mood intervention on self- and reward-bias using a repeated measures analysis of variance (ANOVA) with three within-subjects factors—mood (neutral vs. negative music intervention), task (self vs. reward), and association (self or high reward vs. stranger or low reward). All multiple comparisons were corrected using Holm–Bonferroni corrections for α = .05 (Holm, 1979); (b) conducted correlation analyses across sessions and tasks to assess how the valence of the negative mood shaped the responses to stimuli associated with different people and reward values, across individuals. We assumed that the more negative the mood state after the intervention, the greater any change in the self- and reward-bias effects. We hypothesized that the shift would be greatest in the self and high-reward conditions, whereas the stranger-associated and low-reward-associated stimuli should be less affected (e.g., if they benefit less from outward-facing attention to the stimuli).
Data analyses were based on the data for the correct responses only. Responses faster than 200 ms were also excluded from data analyses (less than 5% of trials were excluded). Mean scores in RTs were applied in data analyses.
Results
The effect of negative mood intervention on mood state
We conducted a pairwise t test to assess differences in reported mood scores between the neutral and negative mood induction sessions. There was a significant difference in mood status between two sessions, t(16) = 11.06, p < .0001 (three participants who did not report mood status based on the instruction were excluded from data analysis). The mood state was more negative after the negative intervention than after the neutral intervention. In order to assess the absolute level of mood state in each session (positive or negative), two simple t tests (against the score “4” indicating neutral mood status) were conducted for each session. The analysis showed a negative mood state after the negative intervention (M = 2.88, SD = ±0.93), t(16) = −4.97, p < .0001, along with a positive mood state after the neutral intervention (M = 5.41, SD = ±0.62), t(16) = 9.41, p < .0001.
The data indicated that our procedure was successful in modulating the mood of participants. Next we assessed how negative mood induction influenced the self- and reward-biases in the matching task. Is there a different effect of negative mood on self- and reward-biases?
An initial overall ANOVA in RTs indicated a borderline interaction between association and response (match vs. mismatch trials), F(1,19) = 4.08, p = .058, η2 = .19. Accordingly the data were analysed by considering effects on match and mismatch trials separately. Note that different processes are likely to be involved on these trial types, including the additional process of rejecting the matching associations on mismatch trials (see Wang et al., 2016).
RTs for match pairs: Negative mood intervention on self- and reward-biases—common effects
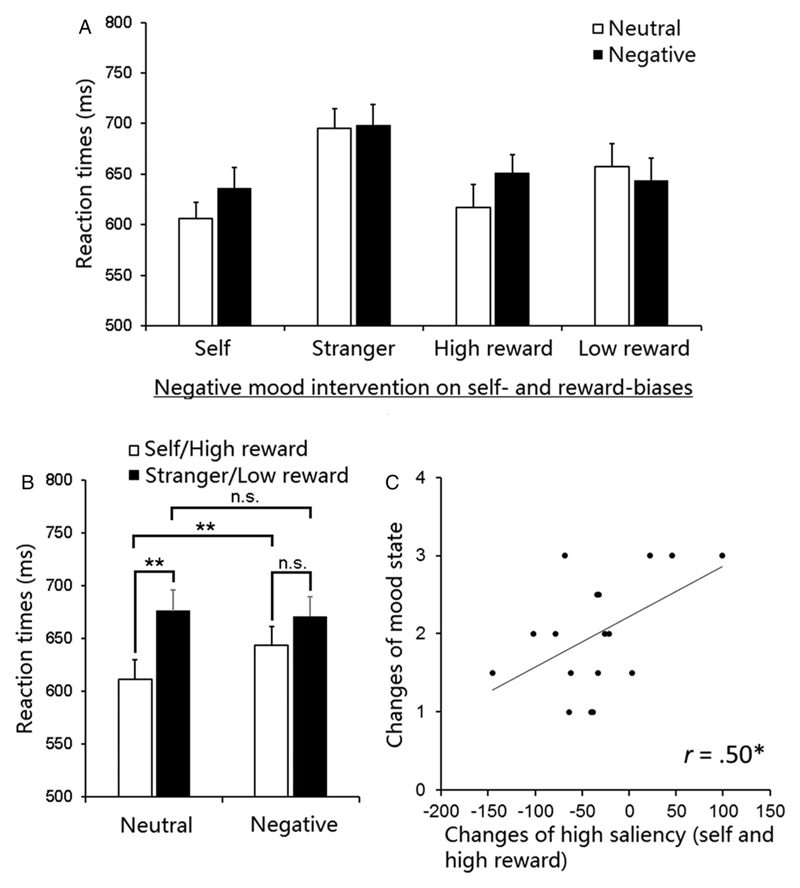
We conducted a three-way ANOVA on RTs for matched pairs with the factors being association (self or high reward vs. stranger or low reward), task (self vs. reward), and mood intervention (negative vs. neutral). The analysis showed a significant main effect of association (self or high reward vs. stranger or low reward), F(1, 19) = 20.19, p < .0001, η2 = .52; there were faster responses to the shapes associated with self and high reward than to the shapes associated with stranger and low reward, reflecting general biases to “high saliency” stimuli (self and high reward). There was a significant main effect of task, F (1, 19) = 5.28, p = .03, η2 = .22, reflecting faster responses to the reward than to the personal association task. The main effect of mood was not significant, F = 1.71, p = .21. There was, however, a significant interaction between association and mood (neutral vs. negative), F(1, 19) = 4.65, p = .04, η2 = .20 (Figures 1A and 1B). No other significant interactions involving mood and task were observed, Fs < 0.70, ps > .41, indicating that negative mood induction exerted a comparable overall effect on the self- and reward-biases.
Figure 1. Performance in reaction times (RTs).
(A) RTs as a function of task (self vs. reward), mood (neutral mood intervention vs. negative mood intervention), and association (self or high reward vs. stranger or. low reward). (B) The effect of negative mood intervention on self- and reward-biases. (C) Correlation between (i) shifts in the valence of mood after the negative music (compared with the neutral music) and (ii) changes in the combined responses to self- and high-reward-associated shapes in RTs across the two sessions. Error bars represent standard errors.
To examine the interaction between association and mood, we conducted pairwise t tests for the two mood intervention sessions. In the neutral mood session there was a strong overall advantage across the self- and high-reward conditions than across the stranger- and low-reward conditions, t(19) = −4.67, p < .0005. This is consistent with prior research (Sui et al., 2012). The overall self- and high-reward advantage remained after the negative mood induction—but in this case this was a marginal effect, t(19) = −2.11, p = .05 (Figure 1B). The interaction reflects the smaller self-/high-reward-bias after negative than after neutral mood induction. The reduced advantage was due to slower responses to self- and high-reward-associated shapes after negative mood intervention (compared to the neutral mood session), t(19) = −2.71, p = .01, but there was no effect of mood intervention on responses to stranger- and low-reward-associated shapes, t(19) = 0.35, p = .73 (Figure 1B).
To assess the relations between the high-saliency bias (self- and high-reward) and the change in mood more directly, we correlated shifts in the valence of mood after the negative mood induction procedure (compared with the neutral mood procedure) with shifts in the biases to high-saliency stimuli (combining the self- and high-reward associations) relative to low-saliency stimuli (combining the stranger and low-reward associations).2 There was a reliable correlation between the change in mood and the changes in the responses to high-saliency stimuli across the two sessions, r (16) = .50, p = .04; the greater the drop in mood, the stronger the shifts in high-saliency stimuli (Figure 1C).
RTs for mismatch pairs
A three-way ANOVA with mood intervention (neutral vs. negative music intervention), task (self vs. reward), and association (self or high-reward vs. stranger vs. low-reward) failed to show any significant effects, Fs < 2.05, ps > .16.
d′
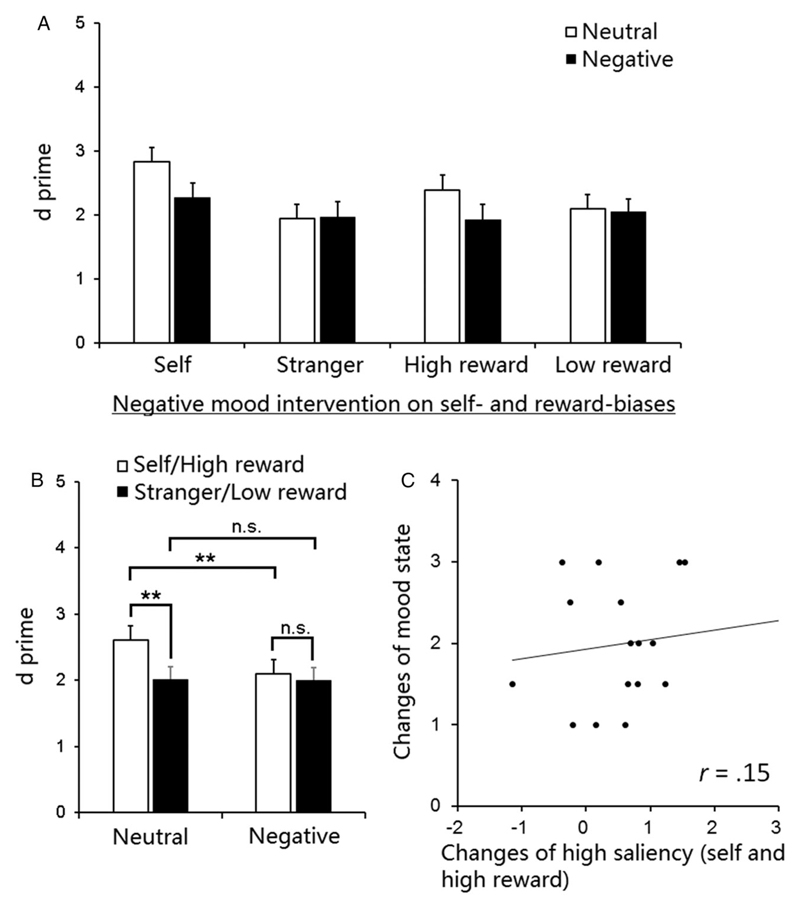
The analysis for d′ demonstrated a significant main effect of association, F(1, 19) = 7.99, p = .01, η2 = .30; there were larger d′ values for shapes associated with self and high reward than for the shapes associated with stranger and low reward, reflecting self- and high-reward advantage effects in perceptual sensitivity. The main effects of mood and task were not significant, F(1, 19) = 2.98 and 2.45, p = .10 and .13. In line with the RT results, the effects were modulated by negative mood intervention. There was a significant interaction between association and mood, F(1, 19) = 4.47, p < .05, η2 = .19 (Figures 2A and 2B). For the neutral mood intervention there was a reliable overall advantage across the self- and high-reward conditions (compared with the stranger- and low-reward conditions), t(19) = 3.36, p < .005. In contrast, the combined self- and high-reward advantage effects were eliminated after negative mood induction, p > .56 (Figure 2B). This reflected reduced sensitivity of shape discrimination to self- and high-reward-associated shapes after negative mood intervention compared to after neutral mood intervention, t(19) = 2.47, p = .02, but there was no effect of mood intervention in responses to stranger- and low-reward-associated shapes between two sessions, p = .93 (Figure 2B). There were no significant interactions involving mood, Fs < 0.27, ps > .61.
Figure 2. Performance in d′.
(A) d′ as a function of task (self vs. reward), mood (neutral mood intervention vs. negative mood intervention), and association (self or high reward vs. stranger or low reward). (B) The effect of negative mood intervention on self- and reward-biases. (C) Correlation between (i) shifts in the valence of mood after the negative music (compared with the neutral music) and (ii) changes in the combined d′ in self- and high-reward-associated shapes in d′ across the two sessions. Error bars represent standard errors.
Unlike the RT results, there were no reliable correlations between the change in individual mood states and the alteration in the combined d′ in high-saliency items (averaged across the self- and the high-reward conditions) across the two sessions, r(16) = .15, p = .57.
Similar to the ANOVA results for the RT data, the d′ results showed that inducing a negative mood reduced both the self- and the reward-advantages in shape sensitivity. The effect was due to sensitivity to self- and high-reward-associated stimuli dropping after a negative mood was induced, while there was no effect on the stranger- and low-reward-associated stimuli. In this case, the drop in self- and reward-biases was not directly related to the absolute drop in mood across individuals. There was also no relation between how the mood shift affected the self-bias and the reward-bias effects.
Discussion
Following prior research (Sui et al., 2012), we confirmed substantial self- and reward-biases on RTs and perceptual sensitivity in simple perceptual matching. Subsequently we had participants listen to neutral or negative music as well as receiving neutral or negative statements. The negative mood intervention was found to reduce the self- and reward-biases, in both cases due to effects on the self- and high-reward conditions (with the stranger and low-reward conditions being unchanged). The decrease in the bias effects did not interact with the type of bias, consistent with common effects on both self- and reward-biases. Furthermore, the drop in mood across participants was correlated with the reductions in the combined biases on RTs (though this did not hold for d′).
The self- and reward-biases found in the perceptual matching task at least partly reflect changes in the perceptual processing of stimuli (e. g., as the biases interact with perceptual variables such as stimulus degradation; Sui et al., 2012). There are also effects based on associative memory for the relations between the shape and the label. For example, Wang et al. (2016) report carry-over effects from the memory for (a) an association between a self-label and (b) the ability to respond to new associations to the same shape. An initial self-association disrupts responses to new associations to the same shapes. The biases on perceptual matching can also reflect increased attention to the self and high-reward stimuli, as shown in studies requiring self- and other-associated targets to be selected and distractors with the opposite association to be ignored (see Sui, Liu, et al., 2013; see also Anderson et al., 2011, for data on the effects of reward). We can account for the present data by suggesting that the two bias effects are dependent on the efficient allocation of attention to external stimuli associated with either the self or the high reward (Anderson et al., 2011; Sui, Liu, et al., 2013). This enhances both perception and memory. The effect of inducing a negative mood may be to turn attention towards internal states rather than to the external stimulus (Paulus, 2007), and this will in turn decrease the self- and high-reward advantages on perception and memory normally induced by the attentional saliency of the stimuli.
Other accounts are also possible, though. For example, one alternative is that both self- and high-reward associations reflect the emotional valence linked to the stimulus—with these associations generating a positive emotional response to the associated stimuli. The positive emotional response may enhance processing of the associated stimulus, speeding perceptual matches to self- and high-reward items. The effect of the negative mood induction then may be to reduce any positive emotional response, which in turn reduces the self- and high-reward advantages. Converging evidence for there being associations between emotion and self and between reward and emotion comes from Ma and Han (2010). These authors showed that evaluating negative personality traits in relation to the self reduced the self- advantage effect in face recognition. Similar effects have also been reported on memory (e.g., Herbert, Pauli, & Herbert, 2011). Going beyond these previous studies, we provide evidence that task-irrelevant modulation (irrelevant negative mood intervention) rather than task-relevant modulation (trait evaluation) can affect biases in perceptual matching. In particular by using a common association procedure for both the self- and reward we showed that both self- and reward-biases decrease with a common mood induction variable. Also, averaging across the biases, we found that changes in self- and reward-biases were correlated with changes in the induced mood.
A refinement on the account in terms of changes in the emotional response to stimuli is that the results reflect a “polarity correspondence effect”, which occurs when stimuli are mapped onto a marked axis to support decision making. For example, we might imagine that self and other stimuli, and high- and low-reward stimuli, are respectively mapped onto opposite ends of a decision axis, with self-related stimuli and stimuli related to high reward mapped onto the marked end of the axis. This marked end can be coded as positive and the other end as negative. It follows that positive/neutral mood may overlap with self/high-reward stimuli, and negative mood with other/low-reward stimuli. The responses to stimuli corresponding to congruent positive polarity (associated with self/high reward, neutral mood induction) are facilitated compared to those to stimuli corresponding to incongruent polarities (associated with self/high-reward, negative mood induction), but there was no effect from congruent negative polarity (stimuli associated with stranger/low reward, negative mood induction; see Lakens, 2012).
In the light of these arguments it is interesting to review the finding that changes in mood state directly correlated with reductions in the bias to high-saliency stimuli (self and high reward), for RTs but not for the measure of perceptual discriminability (d′). For example, consider the argument that negative mood affected attention, and through this effects on perception and memory were changed. We may assume that the d′ results more reflect perception than memory, while RTs may reflect both stages of processing. It would then follow that the mood induction was more directly related to changes in memory than perception, and so correlations emerged for RTs rather than d′. Effects on d′, and perception, may reflect more a step drop in mood. Similar arguments may also be made in relation to claims that effects of negative mood induction stemmed from changes in the emotional valence and “polarity correspondence” of the stimuli—provided we assume that emotion and polarity correspondence modulate memory more than attention.
As well as providing evidence for factors that potentially drive the self- and high-reward-biases, the results are also informative about the relations between self and reward effects themselves. In particular, the matching effects of the negative mood state on self- and reward-biases fits with the idea that these two effects are at least partly related. This too extends prior work that shows that self- and reward-bias effects both interact with the contrast of the stimulus (Sui et al., 2012). We suggest that self-bias effects can include one component that relates to the inherently high reward value linked to self-associated stimuli.
Overall we note that there is little research directly examining the relations between mood, self, and reward, which form three crucial topics for both behavioural and neuroscience studies. By integrating a simple associative learning approach with a mood intervention procedure, we have been able to study the three factors within a single study, showing that mood modulates the self- and reward-biases.
Acknowledgments
This work was supported by grants from the Economic and Social Research Council [grant number ES/J001597/1, UK]; The Wellcome Trust [grant number WT106164MA]; and the European Research Council [grant number Pepe Grant 323883].
Footnotes
Our prior experience indicates that showing participants the stimuli before the perceptual matching task has little effect on performance.
Given that we failed to find any differences between the effects of mood induction on self- and reward-biases, we averaged performance, respectively, across the self- and high-reward conditions and across the stranger- and low-reward conditions, in order to achieve the most stable data for the correlations.
References
- Anderson BA, Laurent PA, Yantis S. Value-driven attentional capture. Proceedings of the National Academy of Sciences of the United States of America. 2011;108:10367–10371. doi: 10.1073/pnas.1104047108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blood AJ, Zatorre RJ. Intensely pleasurable responses to music correlate with activity in brain regions implicated in reward and emotion. Proceedings of the National Academy of Sciences of the United States of America. 2001;98:11818–11823. doi: 10.1073/pnas.191355898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chiew KS, Braver TS. Positive affect versus reward: emotional and motivational influences on cognitive control. Frontiers in Psychology. 2011;2:279. doi: 10.3389/fpsyg.2011.00279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clark DM. On the induction of depressed mood in the laboratory: Evaluation and comparison of the Velten and musical procedures. Advances in Behaviour Research and Therapy. 1983;5:27–49. [Google Scholar]
- Cunningham SJ, Brady-Van den Bos M, Turk DJ. Exploring the effects of ownership and choice on self-memory biases. Memory. 2011;19:449–461. doi: 10.1080/09658211.2011.584388. [DOI] [PubMed] [Google Scholar]
- Cunningham SJ, Turk DJ, Macdonald LM, Macrae CN. Yours or mine? Ownership and memory. Consciousness and Cognition. 2008;17:312–318. doi: 10.1016/j.concog.2007.04.003. [DOI] [PubMed] [Google Scholar]
- Decety J, Sommerville JA. Shared representations between self and other: A social cognitive neuroscience view. Trends in Cognitive Science. 2003;7:527–533. doi: 10.1016/j.tics.2003.10.004. [DOI] [PubMed] [Google Scholar]
- Eshel N, Roiser JP. Reward and punishment processing in depression. Biological Psychiatry. 2010;68:118–124. doi: 10.1016/j.biopsych.2010.01.027. [DOI] [PubMed] [Google Scholar]
- Grimm S, Ernst J, Boesiger P, Schuepbach D, Hell D, Boeker H, Northoff G. Increased self-focus in major depressive disorder is related to neural abnormalities in subcortical-cortical midline structures. Human Brain Mapping. 2009;30:2617–2627. doi: 10.1002/hbm.20693. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Henriques JB, Davidson RJ. Decreased responsiveness to reward in depression. Cognition and Emotion. 2000;14:711–724. [Google Scholar]
- Herbert C, Pauli P, Herbert BM. Self-reference modulates the processing of emotional stimuli in the absence of explicit self-referential appraisal instructions. Social Cognitive and Affective Neuroscience. 2011;6:653–661. doi: 10.1093/scan/nsq082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hervás G, Vázquez C. Low spirits keep rewards subdued: Decreases in sensitivity to reward and vulnerability to dysphoria. Behavior Therapy. 2013;44:62–74. doi: 10.1016/j.beth.2012.07.003. [DOI] [PubMed] [Google Scholar]
- Holm S. A simple sequentially rejective multiple test procedure. Scandinavian Journal of Statistics. 1979;6:65–70. [Google Scholar]
- Lakens D. Polarity correspondence in metaphor congruency effects: Structural overlap predicts categorization times for bipolar concepts presented in vertical space. Journal of Experimental Psychology: Learning, Memory, and Cognition. 2012;38:726–736. doi: 10.1037/a0024955. [DOI] [PubMed] [Google Scholar]
- Ma Y, Han S. Why respond faster to the self than others? An implicit positive association theory of self advantage during implicit face recognition. Journal of Experimental Psychology: Human Perception and Performance. 2010;36:619–633. doi: 10.1037/a0015797. [DOI] [PubMed] [Google Scholar]
- Miller J. Divided attention: Evidence for coactivation with redundant signals. Cognitive Psychology. 1982;14:247–279. doi: 10.1016/0010-0285(82)90010-x. [DOI] [PubMed] [Google Scholar]
- Mordkoff JT, Danek RH. Dividing attention between color and shape revisited: Redundant targets coactivate only when parts of the same perceptual object. Attention, Perception & Psychophysics. 2011;73:103–112. doi: 10.3758/s13414-010-0025-2. [DOI] [PubMed] [Google Scholar]
- Northoff G, Bermpohl E. Cortical midline structures and the self. Trends in Cognitive Science. 2004;8:102–107. doi: 10.1016/j.tics.2004.01.004. [DOI] [PubMed] [Google Scholar]
- Northoff G, Hayes DJ. Is our self nothing but reward? Biological Psychiatry. 2011;69:1019–1025. doi: 10.1016/j.biopsych.2010.12.014. [DOI] [PubMed] [Google Scholar]
- Padmala S, Pessoa L. Motivation versus aversive processing during perception. Emotion. 2014;14:450–454. doi: 10.1037/a0036112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paulus MP. Decision-making dysfunctions in psychiatry—altered homeostatic processing? Science. 2007;318:602–606. doi: 10.1126/science.1142997. [DOI] [PubMed] [Google Scholar]
- Pignatiello MF, Camp CJ, Rasar LA. Music mood induction: An alternative to the Velten technique. Journal of Abnormal Psychology. 1986;94:51–63. doi: 10.1037//0021-843x.95.3.295. [DOI] [PubMed] [Google Scholar]
- Pyszczynksi T, Hamilton J, Herring F, Greenberg J. Depression self-focused attention and the negative memory bias. Journal of Personality and Social Psychology. 1989;57:351–357. [Google Scholar]
- Salovey P. Mood-induced self-focused attention. Journal of Personality and Social Psychology. 1992;62:699–707. doi: 10.1037//0022-3514.62.4.699. [DOI] [PubMed] [Google Scholar]
- Samson D, Apperly IA, Chiavarino C, Humphreys GW. Left temporoparietal junction is necessary for representing someone else’s belief. Nature Neuroscience. 2004;7:499–500. doi: 10.1038/nn1223. [DOI] [PubMed] [Google Scholar]
- Soto D, Funes MJ, Guzman-Garcia A, Warbrick T, Rotshtein P, Humphreys GW. Pleasant music overcomes the loss of awareness in patients with visual neglect. Proceedings of the National Academy of Sciences of the United States of America. 2009;106:6011–6016. doi: 10.1073/pnas.0811681106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sui J, He X, Humphreys GW. Perceptual effects of social salience: Evidence from self-prioritization effects on perceptual matching. Journal of Experimental Psychology, Human Perception and Performance. 2012;38:1105–1117. doi: 10.1037/a0029792. [DOI] [PubMed] [Google Scholar]
- Sui J, Humphreys GW. The integrative self: How self-reference integrates perception and memory. Trends in Cognitive Sciences. 2015;19:719–728. doi: 10.1016/j.tics.2015.08.015. [DOI] [PubMed] [Google Scholar]
- Sui J, Liu M, Mevorach C, Humphreys GW. The salient self: The left intra-parietal sulcus responds to social as well as perceptual-salience after self-association. Cerebral Cortex. 2013a;25:1060–1068. doi: 10.1093/cercor/bht302. [DOI] [PubMed] [Google Scholar]
- Sui J, Rotshtein P, Humphreys GW. Coupling social attention to the self forms a network for personal significance. Proceedings of the National Academy of Sciences of the United States of America. 2013b;110:7607–7612. doi: 10.1073/pnas.1221862110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sui J, Yankouskaya A, Humphreys GW. Super-capacity and violations of race independence for self- but not for reward-associated stimuli. Journal of Experimental Psychology, Human Perception and Performance. 2015;41:441–452. doi: 10.1037/a0038288. [DOI] [PubMed] [Google Scholar]
- Townsend JT, Eidels A. Workload capacity spaces: A unified methodology for response time measures of efficiency as workload is varied. Psychon Bulletin & Review. 2011;18:659–681. doi: 10.3758/s13423-011-0106-9. [DOI] [PubMed] [Google Scholar]
- Uddin LQ, Lacoboni M, Lange C, Keenan JP. The self and social cognition: The role of cortical midline structures and mirror neurons. Trends in Cognitive Science. 2007;11:153–157. doi: 10.1016/j.tics.2007.01.001. [DOI] [PubMed] [Google Scholar]
- Velten E. A laboratory task for induction of mood states. Behavior Research and Therapy. 1968;6:473–482. doi: 10.1016/0005-7967(68)90028-4. [DOI] [PubMed] [Google Scholar]
- Wang H, Humphreys GW, Sui J. Expanding and retracting from the self: Gains and costs in switching self-associations. Journal of Experimental Psychology: Human Perception and Performance. 2016;42:247–256. doi: 10.1037/xhp0000125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Westermann R, Spies K, Stahl G, Hesse FW. Relative effectiveness and validity of mood induction procedures: A meta-analysis. European Journal of Social Psychology. 1996;26:557–580. [Google Scholar]
- Wood JV, Saltzberg JA, Goldsamt LA. Does affect induce self-focused attention? Journal of Personality and Social Psychology. 1990;58:899–908. doi: 10.1037/0022-3514.58.5.899. [DOI] [PubMed] [Google Scholar]