Abstract
Although drugs such as barbiturates and benzodiazepines are often used for the treatment of insomnia, they are associated with various side effects such as habituations, tolerance and addiction. Alternatively, natural products with minimal unwanted effects have been preferred for the treatment of acute and/or mild insomnia, with additional benefits of overall health-promotion. Basic and clinical researches on the mechanisms of action of natural products have been carried out so far in insomnia treatments. Recent studies have been focusing on diverse chemical components available in natural products, with an interest of developing drugs that can improve sleep duration and quality. In the last 15 years, our co-workers have been actively looking for candidate substances from natural products that can relieve insomnia. This review is, therefore, intended to bring pharmacological data regarding to the effects of natural products on sleep duration and quality, mainly through the activation of GABAA receptors. It is imperative that phytochemicals will provide useful information during electroencephalography (EEG) analysis and serve as an alternative medications for insomnia patients who are reluctant to use conventional drugs.
Keywords: Insomnia, Sleep, Natural products, Pharmacological mechanisms, Electroencephalography (EEG)
AN OVERVIEW OF INSOMNIA
Most people often or chronically experience insomnia characterized by difficulty in falling asleep, overnight loss of sleep, trouble to resume sleep, waking up too early, unable to refreshed after sleep and loss of working time due to tiredness and faintness (National Sleep Foundation, USA). Sleep is vital to maintain mood, memory, cognitive function by restoring the majority of human body systems through endocrine and immune functions. Therefore, sleep is one of the most instinctive and essential physiological demand for normal life such as maintaining health and mental stability. Meanwhile, humans may suffer from various sleep disorders, including insomnia, hypersomnia, narcolepsy, and sleep apnea (Jacobson et al., 2017).
On the other hand, insomnia has been associated with psychiatric conditions, unhealthy sleep habits, specific excitatory substances, and/or certain biological factors. Moreover, medical conditions with comorbidities may aggravate these insomnia related abnormalities (Starks et al., 2018).
So far, studies have been focusing on various molecules leading into insomnia. Many of these molecules involved in sleep-wake regulation are produced by specific brain regions with widespread projections. Research findings have been largely interpreted within the context of hyperarousal hypothesis. For instance, insomnia patients with GABA in the occipital cortex has been reported to be consistent with the hyperarousal model of insomnia. In addition, emotional and cognitive systems lead to suppression of sleep-promoting regions such as the ventrolateral preoptic area (VLPO) (Wang and Liu, 2016; Bourcier et al., 2018). In addition, orexin/hypocretin neurons of the lateral hypothalamus project to all of the sleep/insomnia arousal-promoting centers in the brainstem and hypothalamus thereby reinforcing their activity to play on important roles in sleep. The pathophysiological understanding of insomnia may provide important information regarding how, and under what conditions, the disorder develops and is maintained as well as potential targets for prevention and treatment (Jacobson et al., 2017).
ANIMAL SURGERY & EEG RECORDING
Rats were anesthetized with pentobarbital (50 mg/kg, i.p.) and the transmitter was implanted for recording EEG via telemetry as described earlier (Sanford et al., 2006). Briefly, the body of the transmitter was subcutaneously implanted just posterior to the scapula with three sutures for stabilization. The transmitters led subcutaneously to the skull, and the bare ends were placed in contact with the dura through holes in the skull. The electrodes were anchored to the skull with screws and dental cement. All of the surgical procedures were performed stereotaxically under aseptic conditions (Yang et al., 2011b; Perentos et al., 2017).
For telemetric recording of signals from cortical EEG, the gain of transmitters was set at −0.5/+0.5 volts per units ×2 with raw signals ranging from 0.5 to 0.0 Hz and these signals were processed by Data Sciences analog converter and routed to an analog-to-digital (AD) converter (Eagle PC30, Data Sciences International). The AD converter digitized the EEG and activity signals; subsequently data were transferred to a computer and graphically displayed. An on-line fast Fourier transformation (FFT) analyzed EEG data and generated power density values from 0.0 to 20.0 Hz at a resolution of 0.5 Hz. The FFT data were further averaged between 0 to 20 Hz at 10-s intervals. The sleep data and FFT results were saved to the hard disk every 10 s for additional off-line analysis. Movements of the animal in relation to the telemetry receiver generated transistor-transistor logic pulses that were collected and counted as a measure of activity. The signal of EEG was measured for 6 hours between 11:00 am and 5:00 pm. Each group has 5–6 rats (Hu et al., 2013).
Time elapsed in wakefulness, NREM sleep or REM sleep was determined from digitized data within 10 s using the animal sleep analysis software Sleep-Sign 2.1 (Kissei Comtec, Matsumoto, Japan). Briefly, the software identifies wakefulness as a high-frequency with low-amplitude of EEG whereas in NREM sleep, it showed spindles interspersed with slow waves compared with REM sleep characterized by δ-waves (0.75 to 4.0 Hz) and θ-wave activity (5.0 to 9.0 Hz) with peak value at 7.5 Hz.
MOLECULAR TARGETS FOR INSOMNIA THERAPY
Several efficacious pharmacological treatments for insomnia target to various aspects of identified pathophysiologic processes. For instance, GABA is released from the terminal of specific inhibitory neurons and then it binds to its receptors, thereby enhancing chloride influx and facilitates GABAA-ergic transmission (Olsen, 1981; Ticku and Maksay, 1983). This effect is supported by benzodiazepines and barbiturates by their agonistic effects on the GABAA receptors. Thus, their binding to the allosteric site of the receptors enhances the affinity of the GABA-binding site. However, four different types of GABAA receptor subunits have been described, each of which encloses different membranes. Likewise, the critical step in GABA biosynthesis is the decarboxylation of glutamate by glutamic acid decarboxylase (GAD), which exists in two different isoforms, GAD65 and GAD67. The level of GAD65 and GAD67 is reported to be up-regulated in the GABAA-ergic interneurons. With this intricate nature, GABAA receptors have been known to play an important role in the modulation of barbiturate-induced sleeping through interaction with GABAA-ergic systems (Doghramji, 2006). GABA-benzodiazepine receptor agonists which are generally effective in the treatment of insomnia can promote sleep by enhancing the widespread function of GABA. This suggest that new compounds can be developed for specific molecular targets with known sleep-related actions. Apart from this, non-GABAA-chloride channel receptor complex agonist such as melatonin (MT) receptor agonist, 5-HT1A receptor agonist, orexin receptor agonist, adenosine receptor agonist and histamine receptor antagonist have been suggested for the treatment of insomnia (Dujardin et al., 2018). Benzodiazepines are currently the most well-known and most frequently prescribed hypnotic medications, although their use in recent years is being replaced by newer non-benzodiazepine hypnotic drugs, so-called “z-drugs” such as eszopiclone, zolpidem and zaleplon. As these non-benzodiazepine medications are generally believed to be better and safer than earlier generations of sedatives, they have still generated some controversy and discussion regarding side-effects (Schwartz et al., 2017).
The possible role of serotonin (5-HT) in human sleep disorders has been considered. Ursin provides an excellent overview of the evolving concept of the role of serotonin (5-HT) in sleep, supporting the sleep-promoting effects (Ursin, 2002). The role of specific receptor subtypes in sleep-wake regulation has been focused. Gronli reported the effect of certain drugs affecting 5-HT1A and 5-HT1B receptors, which have affinity at the pre- and post-synaptic receptors, later including inhibitory auto-receptors on serotonin neurons, themselves (Grønli et al., 2007). In particular, 5-HT1A/1B, plays a crucial role in regulating serotonin transmission in the brain. Recent studies have also suggested a role for the 5-HT1B receptor in depression, anxiety and sleep. 5-HT1B antagonists reduce the latency to onset of anxiolytic behavior and play a role in stress regulation with activity comparable to diazepam (Tatarczynska et al., 2004). However, some antagonists of serotonin may increase non-rapid eye movement (NREM) sleep. The findings are complex, but support the role of 5-HT1A, 5-HT1B and 5-HT7 receptors in REM control (Leiser et al., 2015).
Non-benzodiazepine hypnotics such as diphenhydramine and doxylamine have been used for the treatment of insomnia. The sedative effect of antihistamines is mainly achieved by targeting the histamine receptors in arousal systems. More recently, there has been progress in the development of orexin receptor antagonists for the treatment of insomnia. Orexin systems target on the promotion of arousal of brainstem/hypothalamic arousal centers (Mieda, 2017). In addition to this, other molecular targets such as melatonin and adenosine have been suggested for the treatment of insomnia (Di Bella et al., 2017).
Various compounds, with novel approaches are being evaluated currently as possible insomnia treatments. Currently, Korea FDA is reviewing new applications for innovative sleep-promoting herbs. Clinical indications have been developed for insomnia associated with problem of sleep onset, sleep maintenance, and middle-of-the-night awakenings. Alternative approaches to treating insomnia have included an off-label basis for insomnia, over-the-counter sleep aids, and assorted unregulated substances marketed to enhance sleep. Substances regarded as appropriate hypnotics are those which prevent continuous awakenings, shorten the period of latency for sleep initiation and increase sleep duration, besides displaying low toxicity.
Key physiological measurements indicators of sleep include electroencephalography (EEG) of brain waves, electrooculography (EOG) of eye movements, electromyography (EMG) of skeletal muscle activity. Simultaneous collection of these measurements is called polysomnography, and can be performed in a specialized sleep laboratory (Rundell and Jones, 1990). Sleep researchers also use simplified electrocardiography (ECG) for cardiac activity and actigraphy for motor movements (Jafari and Mohsenin, 2010). This has promoted the search for alternative approaches such as the employment of phythotherapeutic agents. Animal behavioral experiments were done after surgery and agent treatment (Fig. 1).
Fig. 1.
EEG measurement experiment in the rats. 1. To measure EEG changes, the rats were sutured after implantation of the transmitter body on the back of male rats. 2. The electrode is inserted and fixed with dental cement after puncturing the skull. 3. After one week of recovery from surgery, EEG is recorded. 4. The sleep architecture is analyzed based on the EEG record, which is received on the computer.
NATURAL PRODUCTS WHICH HAVE PROVEN TO BE EFFECTIVE FOR SLEEPING FROM OUR LABORATORY
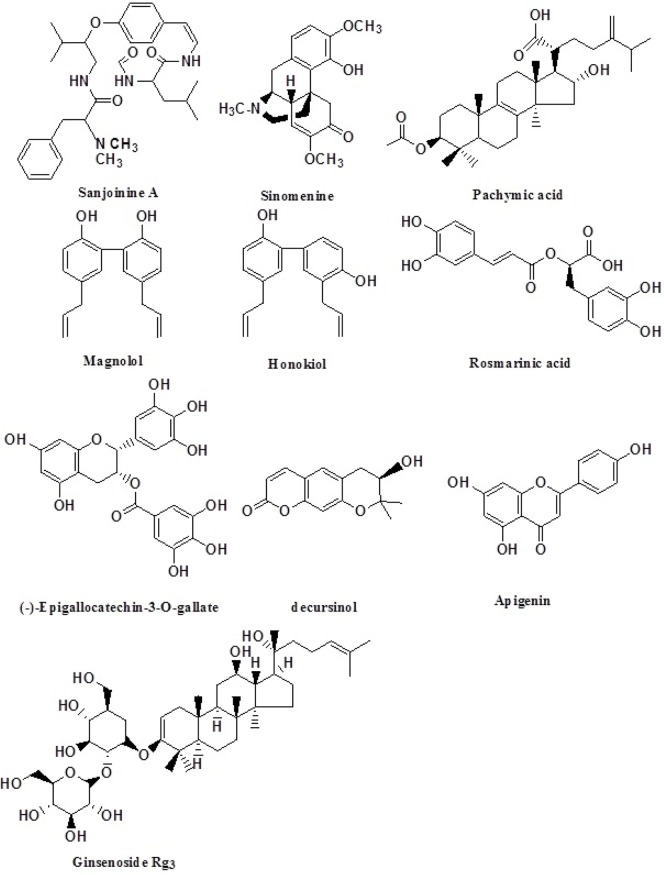
A number of medicinal plants are traditionally endowed with anxiolytic or sedative properties. This has motivated the searching of phythotherapeutic agents, which has been used in animal behavioral experiments performed by surgery (Fig. 1). In this context, there is a lot of information in medicinal plants possessing sedative and hypnotic properties. Here, we describe some natural products studied in our laboratory. These are magnolia (Magnolia officinalis, Magnoliaceae), Semen zizyphi spinosae (Zizyphus jujube Mill. var. spinosa Rhamnaceae), sinomenine (Sinomenium acutum Menispermaceae), decursinol (Angelica gigas, Unbelliferae), rosemary (Perilla frutescens, Lamiaceae), Euphoria longan (Spindaceae), Ginseng (Panax ginseng, Araliacae), EGCG (Epigallocatechin-3-O-gallate, Camellia sinensis), Chrysanthemum morifolium (Asteraceae), Apigenin (Circium japonicum, Asteraceae) and some others that possess sedative effects (Fig. 2).
Fig. 2.
Chemical structures originated from natural products which have been to be effective for sleep from our laboratory.
Magnolia (M. obovate, M. officinalis, Magnoliaceae) has been traditionally used for the treatment of thrombotic stroke, depression, anxiety, and inflammatory and neuronal diseases in oriental countries for a long time (Watanabe et al., 1983; Hirano, 1991; Lo et al., 1994). Biophenolic compounds such as magnolol, honokiol, and obovatol isolated from M. obovate/ and M. officinalis have been found to be anxiolytic and muscle relaxant (Maruyama et al., 1998; Seo et al., 2007; Han et al., 2010). From a previous experiment, it was reported that obovatol has anxiolytic-like effects in animal models, and enhanced pentobarbital-induced sleep suggesting that these effects are involved in GABA/benzodiazepine receptor complex (Seo et al., 2007; Ma et al., 2009b). In addition, research has shown that magnolol and honokiol are the primary active components of M. officinalis and they are positive allosteric modulators of GABAA receptors. In addition, they have shown to increase the density of GABAA receptors that possess alpha subunits and this has been a mechanism followed by diazepam, a benzodiazepine sometimes used to treat insomnia, (Ma et al., 2008, 2009b; Lee et al., 2010). All together, these effects increase GABA activity, which promotes relaxation and reduces anxiety. Magnolol increased the amount of REM and NREM sleep via the GABAA receptors (Chen et al., 2012). Particularly, magnolia bark has been used for human treatment as an anxiolytic, helping to lower anxiety, depression, reduce stress, and facilitating sleep. These effects could be indicators of its positive potential as a natural sleep aiding agent.
Semen zizyphi spinosae (Rhamnaceae, the dried seed of Zizyphus jujube Mill var. spinosa) has been used as a tranquilizer, an anxiolytic and anticonvulsant in oriental countries, and also has been prescribed for the treatment of insomnia and anxiety (Park et al., 2004). It was reported that sanjoinine, which is a major alkaloid compound, and cyclopeptides from Semen zizyphi spinosae have have shown anxiolytic-like effects in the elevated plus-maze, hole-board test and open field test, and these effects may be mediated by GABAA-ergic transmission (Han et al., 2008). Sanjoinine A and cyclopeptide fraction from Semen zizyphi spinosae exert hypnotic effect and/or enhances pentobarbital-induced sleeping behaviors (Ma et al., 2008; Han et al., 2009). Sanjoinine A in combination with muscimol showed synergistic effects on pentobarbital-induced sleeping and increased Cl− influx in a similar way as pentobarbital. Sanjoinine A also showed similar effects with muscimolin potentiating Cl− influx inducing the effects of low dose pentobarbital. This indicated that sanjoinine A might act on GABAA receptor to induce Cl− channel opening, and modulate pentobarbital-induced pharmacological properties like a GABAA receptor agonist (Ma et al., 2007). Moreover, cyclopeptide alkaloid fraction of semen zizyphi spinosae increased pentobarbital-induced sleeping behaviors, through activation of GABA receptor Cl− channels (Ma et al., 2008). However, it has been suggested for high affinity for 5-HT1a and 5-HT2 receptors in in vivo analysis (Yi et al., 2007).
Sinomenine, an alkaloid derived from Sinomenium acutum, is a chief ingredient that has been reported to have a variety of pharmacological properties including anxiolytic effects (Rao et al., 2017). From a recent experiment, sinomenine enhanced pentobarbital-induced sleeping behaviors, and modulate sleep architecture via GABAA-ergic systems in rodents, ultimately increasing NREM sleep.
The most abundant GABAA receptor subunit compositions, α1, β2 and γ2, are related to the hypnotic/sedative effect of GABAA receptors (Rudolph and Möhler, 2006). Previous studies have shown that α1 subunit was associated with sedation (Rudolph and Feiger, 1999; McKernan et al., 2000). Whereas α2/3 subunits were associated with anxiety (Löw et al., 2000; Crestani et al., 2001), α5 subunit was associated with temporal and spatial memory (Collinson et al., 2002; Crestani et al., 2002). Sinomenine non-selectively activated these subunits of GABAA receptors. During our experiment, major subunits of GABAA receptor were over-expressed by sinomenine except for α3 and α5 subunits
Decursinol is one of the major components of Angelica gigas (Umbelliferea) which has been used for a long time as a traditional folk medicine in oriental countries. This herb has been used traditionally for the treatment of psychosomatic disease such as excess stress, anxiety, depression and insomnia. It has been reported that the essential oil components of Angelica gigas exhibited anxiolytic-like effects in rodent tests (Chen et al., 2004). Similarly, Japanese Angelica root extract reversed stress-induced loss of sleep in pentobarbital-induced sleeping in mice through the inhibition of the central noradrenergic or the activation of GABAA receptors (Matsumoto et al., 1998). Decurcinol increased the number of sleeping animals in the sub-hypnotic dosage and modulated sleep architectures, most likely by increasing the protein expression of glutamic acid decarboxylase (GAD65/67) and GABAA receptors subtypes. On the other hand, decurcinol potentiates pentobarbital-induced sleeping behaviors through the activation of GABAA-ergic systems. Altogether suggest that decurcinol, can be useful agent for the treatment of insomnia (Woo et al., 2017).
Rosemary (fromPerilla frutescens, Lamiaceae) has been used as a folk remedy for sedation in oriental countries. So far, several studies have shown that Perilla frutescens has sedative effect (Takeda et al., 2002; Johnston et al., 2006). Rosemary contains a number of phytochemicals, including rosmarinic acid, camphor, caffeic acid, ursolic acid, betulinic acid, carnosic acid and carnosol (Vallverdú-Queralt et al., 2014). Rosmarinic acid belonging to the phenolic compounds is a constituent of Perilla frutescens (Igarashi and Miyazaki, 2013; Rašković et al., 2014). Many of the phenolic compounds originating from plants have been known to affect the GABA-ergic systems (Johnston et al., 2006). In addition, rosmarinic acid inhibited GABA transaminase (GABA-T) in vitro (Awad et al., 2009), and increased the protein expression of GAD65/67 and GABAA receptors subunits except β1 subunit. Rosmarinic acid augmented pentobarbital-induced sleeping behaviors through GABAA-ergic transmission. It is suggested that rosmarinic acid may be useful agent for the treatment of insomnia (Kwon et al., 2017).
Euphoria longan (or Dimocarpus longan, Spindaceae) is commonly consumed as the dried fruit of Euphoria longana. Phytochemicals such as gallic acid, ellastic acid and corilagin (ellagitanin) were identified from the peel, seed and pulp of its fruit (Rang-Kadilok). The pulp of the dried fruit and fresh Longanae Arillus, has been used for the treatment of anxiety and insomnia in Asian countries. The extract of Longane Arillus was proven to have anxiolytic activity (Okuyama et al., 1999). From our experiment, methanol extract of Longane Arillus was proven to prolong sleeping time and reduced pentobarbital-induced sleep onset in rodents through GABAA-ergic systems (Ma et al., 2009b). Methanol extract of Longane Arillus regulated sleep architectures and EEG power spectra in restraint-stressed rats (Ma et al., 2009b).
Ginseng (Panax ginseng, Araliaceae) may be in part related to maintaining sleep and wakefulness, and alter EEG spectra of sleep-wake stage in mice (Ma et al., 2008, 2009a, 2009b; Yang et al., 2011b). Panax quinquefolium (American ginseng) were reported to have sleeping-modulating effects. Saponin fraction of Panax ginseng extract prolonged the duration of hexobarbital-induced sleep in mice (Takagi et al., 1972). In addition, red ginseng extract increased total sleep time and NREM sleep (Ma et al., 2008; Yang et al., 2011a). Majono-sides R2, a major saponin of Panax vietnamensis restored the hypnotic activity of pentobarbital, which was decreased by psychological stresses (Nguyen et al., 1993). Ginsenoside Rg3-standarzied ginseng extract showed anti-stress effects and enhanced sleeping in restraint stressed animals (Kim et al., 2010). There are evidences to suggest that the regulation of GABAA-ergic transmission is one of target for the sedative actions of ginseng (Kimura et al., 1994; Cha et al., 2005; Park et al., 2005). In human study, it has been confirmed that ginseng could improve the quality of sleep (Han et al., 2013). Korea red ginseng increased NREM sleep via GABAA-ergic systems (Lee et al., 2012).
EGCG (Epigallocatechin-3-O-gallate) is a major component of green tea (Camellia sinensis) which is a popular beverage worldwide. However, green tea contains caffeine and EGCG. One is stimulant caffeine, and the other is EGCG which may be sedative. From our experiment, it was interesting that only EGCG from green tea augmented pentobarbital-induced sleeping time through Cl− channel activation (Park et al., 2011). This may suggest that EGCG counteracts caffeine-induced stimulant effects such as hyperactivity, arousal and anxiogenic effects (Park et al., 2010). Anxiolytic effects and enhancement of sleep by EGCG also could be medicated by GABAA-ergic systems (Bae et al., 2002; Campbell et al., 2004; Vignes et al., 2006; Haque et al., 2008; Park et al., 2011).
Chrysanthemum morifolium (Asteraceae). The flower Chrysanthemum morifolium is a medicinal herb that has been used for tea preparation in oriental countries. Chrysanthemumsp contains flavonoids, phenols, cinnamic acids and on. The dry flower of Chrysanthemum morifolium in tea and pillow has been traditionally used for the treatment of insomnia in Korea. Ethanol extract of Chrysanthemum morifolium augments pentobarbital-induced sleeping behaviors through activation of Cl− channels (Kim et al., 2011).
Apigenin (from Circium japonicum, Asteraceae) found in many plants, is a natural product belonging to flavonoids that are the aglycone of several naturally occurring glycosides. Apigenin is a weak ligand for central benzodiazepine receptors in vitro and exerts anxiolytic and slight sedative effects in an animal model (Viola et al., 1995). Apigenin shows second-order positive modulatory activity at GABAA receptors (Campbell et al., 2004; Rosi et al., 2004). It was reported that enhancement of pentobarbital-induced sleep by apigenin is mediated by GABAA receptors through Cl− channel complex activation (Kim et al., 2012).
Others. Some natural products such as Polygalae Radix (3,4,5-trimethoxycinnamic acid), Gastrodiae Rhizoma (4-hydroxybenzaldehyde), Poria cocos (pachymic acid), Rhychophilline (Uncariae Ramulus et Uncus) and Perilla Herba, were studied whether they enhance sleep or not (Lee et al., 2013; Choi et al., 2014; Shah et al., 2014; Choi et al., 2015; Shah et al., 2015; Kwon et al., 2017). From the behavioral and molecular data, those natural products augmented pentobarbital-induced sleep, and modulated sleep architectures of EEG spectra in rodents. The increase of sleep seems to be mediated through GABAA-benzodiazepine receptors Cl− channel complex.
However, cordycepin (Cordyceps militaris/sinensis) increased theta waves power density during NREM sleep. Adenosine receptors (AR) subtypes (A1, A2A and A2B) were over-expressed by cordycepin, and they are showing non-specific AR activation (Hu et al., 2013).
CONCLUSION
Up to date, over the counter sleep aiding agents have been introduced. St. John’s wort (Pipericum perforatum), Kava kava (Piper methysticum), Valerian (Valerinana officinalis) and Passion flower (Passiflora incameta) and other herbs, which have been used for the treatment of insomnia become popular as an alternative medicine. Natural products which have been proven to be effective for sleeping from our laboratory are reviewed. However, preclinical and clinical studies are needed to evaluate the precise effects of natural products for the treatment of insomnia.
Acknowledgments
This research was supported by the National Research Foundation (NRF) grant funded by the Korea government, Ministry of Science, ICT & Future Planning (MRC 2010-0029355).
Footnotes
CONFLICT OF INTEREST
All authors declare no conflict of interest.
REFERENCES
- Awad R, Muhammad A, Durst T, Trudeau VL, Arnason JT. Bioassay-guided fractionation of lemon balm (Melissa officinalis L.) using an in vitro measure of GABA transaminase activity. Phytother Res. 2009;23:1075–1081. doi: 10.1002/ptr.2712. [DOI] [PubMed] [Google Scholar]
- Bae YC, Choi BJ, Lee MG, Lee HJ, Park KP, Zhang LF, Honma S, Fukami H, Yoshida A, Ottersen OP. Quantitative ultrastructural analysis of glycine- and γ-aminobutyric acid-immunoreactive terminals on trigeminal α-and γ-motoneuron somata in the rat. J Comp Neurol. 2002;442:308–319. doi: 10.1002/cne.10092. [DOI] [PubMed] [Google Scholar]
- Bourcier E, Korb-Savoldelli V, Hejblum G, Fernandez C, Hindlet P. A systematic review of regulatory and educational interventions to reduce the burden associated with the prescriptions of sedative-hypnotics in adults treated for sleep disorders. PLoS ONE. 2018;13:e0191211. doi: 10.1371/journal.pone.0191211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Campbell EL, Chebib M, Johnston GA. The dietary flavonoids apigenin and (−)-epigallocatechin gallate enhance the positive modulation by diazepam of the activation by GABA of recombinant GABAA receptors. Biochem Pharmacol. 2004;68:1631–1638. doi: 10.1016/j.bcp.2004.07.022. [DOI] [PubMed] [Google Scholar]
- Cha H-Y, Park J-H, Hong J-T, Yoo H-S, Song S, Hwang B-Y, Eun J-S, Oh K-W. Anxiolytic-like effects of ginsenosides on the elevated plus-maze model in mice. Biol Pharm Bull. 2005;28:1621–1625. doi: 10.1248/bpb.28.1621. [DOI] [PubMed] [Google Scholar]
- Chen C-R, Zhou X-Z, Luo Y-J, Huang Z-L, Urade Y, Qu W-M. Magnolol, a major bioactive constituent of the bark of Magnolia officinalis, induces sleep via the benzodiazepine site of GABAA receptor in mice. Neuropharmacology. 2012;63:1191–1199. doi: 10.1016/j.neuropharm.2012.06.031. [DOI] [PubMed] [Google Scholar]
- Chen CD, Welsbie DS, Tran C, Baek SH, Chen R, Vessella R, Rosenfeld MG, Sawyers CL. Molecular determinants of resistance to antiandrogen therapy. Nat Med. 2004;10:33. doi: 10.1038/nm972. [DOI] [PubMed] [Google Scholar]
- Choi JJ, Kim YS, Kwon YO, Yoo JH, Chong MS, Lee MK, Hong JT, Oh KW. 4-hydroxybenzaldehyde, one of constituents from Gastrodiae rhizoma augments pentobarbital-induced sleeping behaviors and non-rapid eye movement (NREM) sleep in rodents. Nat Prod Sci. 2015;21:219–225. [Google Scholar]
- Choi JJ, Oh E-H, Lee MK, Chung YB, Hong JT, Oh K-W. Gastrodiae Rhizoma ethanol extract enhances pentobarbital-induced sleeping behaviors and rapid eye movement sleep via the activation of GABAA-ergic transmission in rodents. Evid Based Complement Alternat Med. 2014;2014:426843. doi: 10.1155/2014/426843. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Collinson N, Kuenzi FM, Jarolimek W, Maubach KA, Cothliff R, Sur C, Smith A, Otu FM, Howell O, Atack JR. Enhanced learning and memory and altered GABAergic synaptic transmission in mice lacking the α5 subunit of the GABAA Receptor. J Neurosci. 2002;22:5572–5580. doi: 10.1523/JNEUROSCI.22-13-05572.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crestani F, Keist R, Fritschy J-M, Benke D, Vogt K, Prut L, Blüthmann H, Möhler H, Rudolph U. Trace fear conditioning involves hippocampal α5 GABAA receptors. Proc Natl Acad Sci USA. 2002;99:8980–8985. doi: 10.1073/pnas.142288699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crestani F, Löw K, Keist R, Mandelli M-J, Möhler H, Rudolph U. Molecular targets for the myorelaxant action of diazepam. Mol Pharmacol. 2001;59:442–445. doi: 10.1124/mol.59.3.442. [DOI] [PubMed] [Google Scholar]
- Di Bella G, Gualano L, Di Bella L. Melatonin with adenosine solubilized in water and stabilized with glycine for oncological treatment–technical preparation, effectivity and clinical findings. Neuro Endocrinol Lett. 2017;38:465–474. [PubMed] [Google Scholar]
- Doghramji K. The epidemiology and diagnosis of insomnia. Am J Manag Care. 2006;12:S214–S220. [PubMed] [Google Scholar]
- Dujardin S, Pijpers A, Pevernagie D. Prescription drugs used in insomnia. Sleep Med Clin. 2018;13:169–182. doi: 10.1016/j.jsmc.2018.03.001. [DOI] [PubMed] [Google Scholar]
- Grønli J, Fiske E, Murison R, Bjorvatn B, Sørensen E, Ursin R, Portas CM. Extracellular levels of serotonin and GABA in the hippocampus after chronic mild stress in rats. A microdialysis study in an animal model of depression. Behav Brain Res. 2007;181:42–51. doi: 10.1016/j.bbr.2007.03.018. [DOI] [PubMed] [Google Scholar]
- Han H, Ma Y, Eun J-S, Hong J-T, Oh K-W. Anxiolytic-like effects of cyclopeptide fraction alkaloids of Zizyphi Spinosi semen: possible involvement of GABAA receptors. Biomol Ther (Seoul) 2008;16:261–269. doi: 10.4062/biomolther.2008.16.3.261. [DOI] [Google Scholar]
- Han H, Ma Y, Eun JS, Li R, Hong J-T, Lee M-K, Oh K-W. Anxiolytic-like effects of sanjoinine A isolated from Zizyphi Spinosi Semen: possible involvement of GABAergic transmission. Pharmacol Biochem Behav. 2009;92:206–213. doi: 10.1016/j.pbb.2008.11.012. [DOI] [PubMed] [Google Scholar]
- Han HJ, Kim HY, Choi JJ, Ahn S-Y, Lee S-H, Oh K-W, Kim S-Y. Effects of red ginseng extract on sleeping behaviors in human volunteers. J Ethnopharmacol. 2013;149:597–599. doi: 10.1016/j.jep.2013.07.005. [DOI] [PubMed] [Google Scholar]
- Han TH, Lee K, Park JB, Ahn D, Park J-H, Kim D-Y, Stern JE, Lee SY, Ryu PD. Reduction in synaptic GABA release contributes to target-selective elevation of PVN neuronal activity in rats with myocardial infarction. Am J Physiol Regul Integr Comp Physiol. 2010;299:R129–R139. doi: 10.1152/ajpregu.00391.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haque AM, Hashimoto M, Katakura M, Hara Y, Shido O. Green tea catechins prevent cognitive deficits caused by Aβ1–40 in rats. J Nutr Biochem. 2008;19:619–626. doi: 10.1016/j.jnutbio.2007.08.008. [DOI] [PubMed] [Google Scholar]
- Hirano T. Differential pre- and postsynaptic mechanisms for synapic potentiation and depression between a granule cell and a purkinje cell in rat cerebellar culture. Synapse. 1991;7:321–323. doi: 10.1002/syn.890070408. [DOI] [PubMed] [Google Scholar]
- Hu Z, Lee C-I, Shah VK, Oh E-H, Han J-Y, Bae J-R, Lee K, Chong M-S, Hong JT, Oh K-W. Cordycepin increases nonrapid eye movement sleep via adenosine receptors in rats. Evid Based Complement Alternat Med. 2013;2013:840134. doi: 10.1155/2013/840134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Igarashi M, Miyazaki Y. A review on bioactivities of perilla: progress in research on the functions of perilla as medicine and food. Evid Based Complement Alternat Med. 2013;2013:925342. doi: 10.1155/2013/925342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jacobson LH, Hoyer D, Fehlmann D, Bettler B, Kaupmann K, Cryan JF. Blunted 5-HT 1A receptor-mediated responses and antidepressant-like behavior in mice lacking the GABA B1a but not GABA B1b subunit isoforms. Psychopharmacology. 2017;234:1511–1523. doi: 10.1007/s00213-016-4521-5. [DOI] [PubMed] [Google Scholar]
- Jafari B, Mohsenin V. Polysomnography. Clin Chest Med. 2010;31:287–297. doi: 10.1016/j.ccm.2010.02.005. [DOI] [PubMed] [Google Scholar]
- Johnston GA, Hanrahan JR, Chebib M, Duke RK, Mewett KN. Modulation of ionotropic GABA receptors by natural products of plant origin. Adv Pharmacol. 2006;54:285–316. doi: 10.1016/S1054-3589(06)54012-8. [DOI] [PubMed] [Google Scholar]
- Kim C-S, Jo Y-J, Park S-H, Kim H-J, Han J-Y, Hong J-T, Cheong J-H, Oh K-W. Anti-stress effects of ginsenoside Rg 3-standardized ginseng extract in restraint stressed animals. Biomol Ther (Seoul) 2010;18:219–225. doi: 10.4062/biomolther.2010.18.2.219. [DOI] [Google Scholar]
- Kim J-W, Han J-Y, Hong JT, Li R, Eun JS, Oh K-W. Ethanol extract of the flower Chrysanthemum morifolium augments pentobarbital-induced sleep behaviors: involvement of Cl− channel activation. Evid Based Complement Alternat Med. 2011;2011:109164. doi: 10.1155/2011/109164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim J-W, Kim C-S, Hu Z, Han J-Y, Kim SK, Yoo S-K, Yeo YM, Chong MS, Lee K, Hong JT. Enhancement of pentobarbital-induced sleep by apigenin through chloride ion channel activation. Arch Pharm Res. 2012;35:367–373. doi: 10.1007/s12272-012-0218-4. [DOI] [PubMed] [Google Scholar]
- Kimura T, Saunders P, Kim H, Rheu H, Oh K, Ho I. Interactions of ginsenosides with ligand-bindings of GABA(A) and GABA(B) receptors. Gen Pharmacol. 1994;25:193–199. doi: 10.1016/0306-3623(94)90032-9. [DOI] [PubMed] [Google Scholar]
- Kwon YO, Hong JT, Oh K-W. Rosmarinic acid potentiates pentobarbital-induced sleep behaviors and non-rapid eye movement (NREM) sleep through the activation of GABAA-ergic systems. Biomol Ther (Seoul) 2017;25:105–111. doi: 10.4062/biomolther.2016.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Löw K, Crestani F, Keist R, Benke D, Brünig I, Benson JA, Fritschy J-M, Rülicke T, Bluethmann H, Möhler H. Molecular and neuronal substrate for the selective attenuation of anxiety. Science. 2000;290:131–134. doi: 10.1126/science.290.5489.131. [DOI] [PubMed] [Google Scholar]
- Lee C-I, Han J-Y, Hong JT, Oh K-W. 3, 4, 5-Trimethoxycinnamic acid (TMCA), one of the constituents of Polygalae Radix enhances pentobarbital-induced sleeping behaviors via GABA A ergic systems in mice. Arch Pharm Res. 2013;36:1244–1251. doi: 10.1007/s12272-013-0167-6. [DOI] [PubMed] [Google Scholar]
- Lee Y-J, Choi D-Y, Lee YK, Lee YM, Han SB, Kim YH, Kim KH, Nam S-Y, Lee BJ, Kang J-K. 4-O-methylhonokiol prevents memory impairment in the Tg2576 transgenic mice model of Alzheimer’s disease via regulation of β-secretase activity. J Alzheimers Dis. 2012;29:677–690. doi: 10.3233/JAD-2012-111835. [DOI] [PubMed] [Google Scholar]
- Lee YK, Song JK, Choi IS, Jeong JH, Moon DC, Yun YP, Han SB, Oh K-W, Hong JT. Neurotrophic activity of obovatol on the cultured embryonic rat neuronal cells by increase of neurotrophin release through activation of ERK pathway. Eur J Pharmacol. 2010;649:168–176. doi: 10.1016/j.ejphar.2010.09.029. [DOI] [PubMed] [Google Scholar]
- Leiser SC, Li Y, Pehrson AL, Dale E, Smagin G, Sanchez C. Serotonergic regulation of prefrontal cortical circuitries involved in cognitive processing: a review of individual 5-HT receptor mechanisms and concerted effects of 5-HT receptors exemplified by the multimodal antidepressant vortioxetine. ACS Chem Neurosci. 2015;6:970–986. doi: 10.1021/cn500340j. [DOI] [PubMed] [Google Scholar]
- Lo HH, Valentovic MA, Rankin GO, Brown PI. Effect of chemical form, route of administration and vehicle on 3, 5-dichloroaniline-induced nephrotoxicity in the fischer 344 rat. J Appl Toxicol. 1994;14:417–422. doi: 10.1002/jat.2550140606. [DOI] [PubMed] [Google Scholar]
- Ma Y, Han H, Eun JS, Kim H-C, Hong J-T, Oh K-W. Sanjoinine A isolated from Zizyphi Spinosi Semen augments pentobarbital-induced sleeping behaviors through the modification of GABA-ergic systems. Biol Pharm Bull. 2007;30:1748–1753. doi: 10.1248/bpb.30.1748. [DOI] [PubMed] [Google Scholar]
- Ma Y, Kim YB, Nam SY, CHEONG JH, Park SH, Kim HJ, Hong JT, OH KW. Effects of red ginseng extract on sleep architecture and electroencephalogram power spectra in rats. Sleep Biol Rhythms. 2009a;7:78–83. doi: 10.1111/j.1479-8425.2009.00391.x. [DOI] [Google Scholar]
- Ma Y, Ma H, Eun JS, Nam S-Y, Kim Y-B, Hong J-T, Lee M-K, Oh K-W. Methanol extract of Longanae Arillus augments pentobarbital-induced sleep behaviors through the modification of GABAergic systems. J Ethnopharmacol. 2009b;122:245–250. doi: 10.1016/j.jep.2009.01.012. [DOI] [PubMed] [Google Scholar]
- Ma Y, Yun S-R, Nam S-Y, Kim Y-B, Hong J-T, Kim Y, Choi H, Lee K, Oh K-W. Protective effects of sanjoinine A against N-methyl-D-aspartate-induced seizure. Biol Pharm Bull. 2008;31:1749–1754. doi: 10.1248/bpb.31.1749. [DOI] [PubMed] [Google Scholar]
- Maruyama K, Nakamura T, Yoshihara T, Fukutomi J, Sugiyama K, Hattorim K, Ohnuki T, Watanabe K, Nagatomo T. Tamsulosin: assessment of affinity of 3H-prazosin bindings to two α1-adrenoceptor subtypes (α1H and α1L) in bovine prostate and rat heart and brain. Gen Pharmacol. 1998;31:597–600. doi: 10.1016/S0306-3623(98)00048-2. [DOI] [PubMed] [Google Scholar]
- Matsumoto K, Kohno S-I, Qjima K, Tezuka Y, Kadota S, Watanabe H. Effects of methylenechloride-soluble fraction of Japanese angelica root extract, ligustilide and butylidene-phthalide, on pentobarbital sleep in group-housed and socially isolated mice. Life Sci. 1998;62:2073–2082. doi: 10.1016/S0024-3205(98)00182-9. [DOI] [PubMed] [Google Scholar]
- McKernan R, Rosahl T, Reynolds D, Sur C, Wafford K, Atack J, Farrar S, Myers J, Cook G, Ferris P. Sedative but not anxiolytic properties of benzodiazepines are mediated by the GABA A receptor α 1 subtype. Nat Neurosci. 2000;3:587–592. doi: 10.1038/75761. [DOI] [PubMed] [Google Scholar]
- Mieda M. The roles of orexins in sleep/wake regulation. Neurosci Res. 2017;118:56–65. doi: 10.1016/j.neures.2017.03.015. [DOI] [PubMed] [Google Scholar]
- Nguyen M, Nguyen T, Kasai R, Ito A, Yamasaki K, Tanaka O. Saponins from Vietnamese ginseng, Panax vietnamensis Ha et Grushv. Collected in central Vietnam. I. Chem Pharm Bull. 1993;41:2010–2014. doi: 10.1248/cpb.41.2010. [DOI] [PubMed] [Google Scholar]
- Okuyama E, Ebihara H, Takeuchi H, Yamazaki M. Adenosine, the anxiolytic-like principle of the Arillus of Euphoria longana. Planta Med. 1999;65:115–119. doi: 10.1055/s-1999-14055. [DOI] [PubMed] [Google Scholar]
- Olsen RW. GABA-benzodiazepine-barbiturate receptor interactions. J Neurochem. 1981;37:1–13. doi: 10.1111/j.1471-4159.1981.tb05284.x. [DOI] [PubMed] [Google Scholar]
- Park J, Kim SY, Cha G-H, Lee SB, Kim S, Chung J. Drosophila DJ-1 mutants show oxidative stress-sensitive locomotive dysfunction. Gene. 2005;361:133–139. doi: 10.1016/j.gene.2005.06.040. [DOI] [PubMed] [Google Scholar]
- Park K-S, Han J-Y, Moon D-C, Hong JT, Oh K-W. (−)-Epigallocatechin-3-O-gallate augments pentobarbital-induced sleeping behaviors through Cl− channel activation. J Med Food. 2011;14:1456–1462. doi: 10.1089/jmf.2010.1529. [DOI] [PubMed] [Google Scholar]
- Park K-S, Oh JH, Yoo H-S, Lee Y-M, Lee M-K, Hong JT, Oh K-W. (−)-Epigallocatechin-3-O-gallate (EGCG) reverses caffeine-induced anxiogenic-like effects. Neurosci Lett. 2010;481:131–134. doi: 10.1016/j.neulet.2010.06.072. [DOI] [PubMed] [Google Scholar]
- Park KS, Da Lee R, Kang S-K, Han SY, Park KL, Yang KH, Song YS, Park HJ, Lee YM, Yun YP. Neuronal differentiation of embryonic midbrain cells by upregulation of peroxisome proliferator-activated receptor-gamma via the JNK-dependent pathway. Exp Cell Res. 2004;297:424–433. doi: 10.1016/j.yexcr.2004.03.034. [DOI] [PubMed] [Google Scholar]
- Perentos N, Nicol A, Martins A, Stewart J, Taylor P, Morton AJ. Techniques for chronic monitoring of brain activity in freely moving sheep using wireless EEG recording. J Neurosci Methods. 2017;279:87–100. doi: 10.1016/j.jneumeth.2016.11.010. [DOI] [PubMed] [Google Scholar]
- Rao S, Liu S, Zou L, Jia T, Zhao S, Wu B, Yi Z, Wang S, Xue Y, Gao Y. The effect of sinomenine in diabetic neuropathic pain mediated by the P2X 3 receptor in dorsal root ganglia. Purinergic Signal. 2017;13:227–235. doi: 10.1007/s11302-016-9554-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rašković A, Milanović I, Pavlović N, Ćebović T, Vukmirović S, Mikov M. Antioxidant activity of rosemary (Rosmarinus officinalis L.) essential oil and its hepatoprotective potential. BMC Complement Altern Med. 2014;14:225. doi: 10.1186/1472-6882-14-225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosi S, Ramirez-Amaya V, Hauss-Wegrzyniak B, Wenk GL. Chronic brain inflammation leads to a decline in hippocampal NMDA-R1 receptors. J Neuroinflammation. 2004;1:12. doi: 10.1186/1742-2094-1-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rudolph RL, Feiger AD. A double-blind, randomized, placebo-controlled trial of once-daily venlafaxine extended release (XR) and fluoxetine for the treatment of depression. J Affect Disord. 1999;56:171–181. doi: 10.1016/S0165-0327(99)00067-1. [DOI] [PubMed] [Google Scholar]
- Rudolph U, Möhler H. GABA-based therapeutic approaches: GABAA receptor subtype functions. Curr Opin Pharmacol. 2006;6:18–23. doi: 10.1016/j.coph.2005.10.003. [DOI] [PubMed] [Google Scholar]
- Rundell O, Jones R. Polysomnography methods and interpretations. Otolaryngol Clin North Am. 1990;23:583–592. [PubMed] [Google Scholar]
- Sanford LD, Yang L, Liu X, Tang X. Effects of tetrodotoxin (TTX) inactivation of the central nucleus of the amygdala (CNA) on dark period sleep and activity. Brain Res. 2006;1084:80–88. doi: 10.1016/j.brainres.2006.02.020. [DOI] [PubMed] [Google Scholar]
- Schwartz J, Neukirchen M, De Vilder M-C, Hornemann B, Wolf C, Gärtner J, Thomas M. SOP-Depression und Angst in der Palliativmedizin. Der Onkologe. 2017;23:756–763. doi: 10.1007/s00761-017-0260-4. [DOI] [Google Scholar]
- Seo J-J, Lee S-H, Lee Y-S, Kwon B-M, Ma Y, Hwang B-Y, Hong J-T, Oh K-W. Anxiolytic-like effects of obovatol isolated from Magnolia obovata: involvement of GABA/benzodiazepine receptors complex. Prog Neuropsychopharmacol Biol Psychiatry. 2007;31:1363–1369. doi: 10.1016/j.pnpbp.2007.05.009. [DOI] [PubMed] [Google Scholar]
- Shah P, Singh S, Kumar A. Combined effect of hydroethanolic extracts of Murraya koenigii and Phyllanthus niruri leaves on paracetamol and ethanol-induced toxicity in HepG2 cell line. Current Science (00113891) 2015;109:1320–1326. doi: 10.18520/cs/v109/i7/1320-1344. [DOI] [Google Scholar]
- Shah VK, Choi JJ, Han J-Y, Lee MK, Hong JT, Oh K-W. Pachymic acid enhances pentobarbital-induced sleeping behaviors via GABAA-ergic systems in mice. Biomol Ther (Seoul) 2014;22:314–320. doi: 10.4062/biomolther.2014.045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Starks SA, Outlaw F, Graff JC, Likes W, White-Means S, Melaro L, Wicks MN. Quality of life and african american women who are family caregivers: a literature review with implications for psychiatric mental health advanced practice registered nurses. Issues Ment Health Nurs. 2018;39:467–481. doi: 10.1080/01612840.2017.1423427. [DOI] [PubMed] [Google Scholar]
- Takagi K, Saito H, Tsuchiya M. Pharmacological studies of Panax ginseng root: pharmacological properties of a crude saponin fraction. Jpn J Pharmacol. 1972;22:339–346. doi: 10.1254/jjp.22.339. [DOI] [PubMed] [Google Scholar]
- Takeda H, Tsuji M, Inazu M, Egashira T, Matsumiya T. Rosmarinic acid and caffeic acid produce antidepressive-like effect in the forced swimming test in mice. Eur J Pharmacol. 2002;449:261–267. doi: 10.1016/S0014-2999(02)02037-X. [DOI] [PubMed] [Google Scholar]
- Tatarczynska E, Klodzinska A, Stachowicz K, Chojnacka-Wojcik E. Effects of a selective 5-HT1B receptor agonist and antagonists in animal models of anxiety and depression. Behav Pharmacol. 2004;15:523–534. doi: 10.1097/00008877-200412000-00001. [DOI] [PubMed] [Google Scholar]
- Ticku MK, Maksay G. Convulsant/depressant site of action at the allosteric benzodiazepine-GABA receptor-ionophore complex. Life Sci. 1983;33:2363–2375. doi: 10.1016/0024-3205(83)90630-6. [DOI] [PubMed] [Google Scholar]
- Ursin R. Serotonin and sleep. Sleep Med Rev. 2002;6:55–67. doi: 10.1053/smrv.2001.0174. [DOI] [PubMed] [Google Scholar]
- Vallverdú-Queralt A, Regueiro J, Martínez-Huélamo M, Alvarenga JFR, Leal LN, Lamuela-Raventos RM. A compre-hensive study on the phenolic profile of widely used culinary herbs and spices: rosemary, thyme, oregano, cinnamon, cumin and bay. Food Chem. 2014;154:299–307. doi: 10.1016/j.foodchem.2013.12.106. [DOI] [PubMed] [Google Scholar]
- Vignes M, Maurice T, Lanté F, Nedjar M, Thethi K, Guiramand J, Récasens M. Anxiolytic properties of green tea polyphenol (−)-epigallocatechin gallate (EGCG) Brain Res. 2006;1110:102–115. doi: 10.1016/j.brainres.2006.06.062. [DOI] [PubMed] [Google Scholar]
- Viola H, Wasowski C, De Stein ML, Wolfman C, Silveira R, Dajas F, Medina J, Paladini A. Apigenin, a component of Matricaria recutita flowers, is a central benzodiazepine receptors-ligand with anxiolytic effects. Planta Med. 1995;61:213–216. doi: 10.1055/s-2006-958058. [DOI] [PubMed] [Google Scholar]
- Wang ZJ, Liu JF. The molecular basis of insomnia: implication for therapeutic approaches. Drug Dev Res. 2016;77:427–436. doi: 10.1002/ddr.21338. [DOI] [PubMed] [Google Scholar]
- Watanabe K, Watanabe H, Goto Y, Shimizu M, Maeda-Hagiwara M. γ-Butyrolactone enhances the activity of GABA in the gastric acid secretion of anesthetized rats. Jpn J Pharmacol. 1983;33:1163–1169. doi: 10.1254/jjp.33.1163. [DOI] [PubMed] [Google Scholar]
- Woo JH, Ha T-W, Kang J-S, Hong JT, Oh K-W. Potentiation of decursinol angelate on pentobarbital-induced sleeping behaviors via the activation of GABAA-ergic systems in rodents. Korean J Physiol Pharmacol. 2017;21:27–36. doi: 10.4196/kjpp.2017.21.1.27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang J-E, Kim S-Y, Im W-T, Yi T-H. Flavobacterium ginsenosidimutans sp. nov., a bacterium with ginsenoside converting activity isolated from soil of a ginseng field. Int J Syst Evol Microbiol. 2011a;61:1408–1412. doi: 10.1099/ijs.0.025700-0. [DOI] [PubMed] [Google Scholar]
- Yang S-L, Han J-Y, Kim Y-B, Nam S-Y, Song S, Hong JT, Oh K-W. Increased non-rapid eye movement sleep by cocaine withdrawal: possible involvement of A 2A receptors. Arch Pharm Res. 2011b;34:281–287. doi: 10.1007/s12272-011-0214-0. [DOI] [PubMed] [Google Scholar]
- Yi P-L, Lin C-P, Tsai C-H, Lin J-G, Cang F-C. The involvement of serotonin receptors in suanzaorentang-induced sleep alteration. J Biomed Sci. 2007;14:829–840. doi: 10.1007/s11373-007-9197-8. [DOI] [PubMed] [Google Scholar]