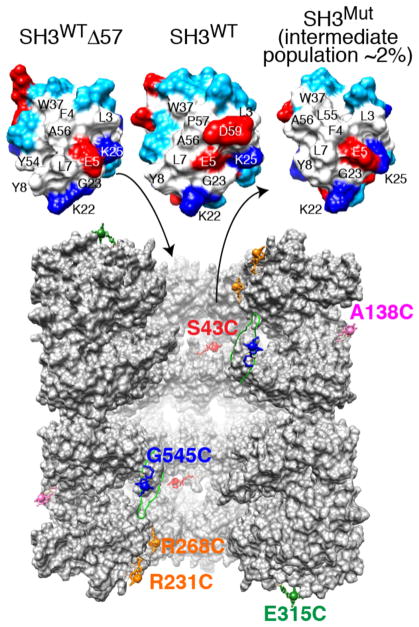
Figure 1.
Fyn SH3 variants and sites of GroEL paramagnetic labeling. Top: molecular surface of the folding intermediate mimetic SH3WTΔ57, native SH3WT, and the SH3Mut folding intermediate. Residue coloring: hydrophobic, white; hydrophilic, cyan; positively charged, dark blue; and negatively charged, red. The largest differences in chemical shift between free and GroEL-bound SH3Mut are observed for residues 2–7, 28, 37, and 50–56 that form a largely hydrophobic surface patch.14 Bottom: vertical slice through the two GroEL cavities showing five subunits for each stacked ring.4 (Note GroEL is depicted at a ~2 times smaller scale than SH3.) The locations of the solvent-accessible engineered cysteine residues used for paramagnetic labeling with EDTA–Mn2+ are indicated. Note that all 14 subunits bear a single paramagnetic label per sample. EDTA was conjugated to GroEL via a disulfide linkage to each Cys mutation to yield a (cysteaminyl–EDTA)–Cys adduct, which is then chelated to either Mn2+ (paramagnetic) or Ca2+ (diamagnetic).