ABSTRACT
Intestinal parasitic infections are a common health problem among Amerindian populations and schistosomiasis represents one of the most prevalent diseases in Maxakali people. The Kato-Katz is the diagnostic method recommended by WHO for epidemiological studies; however, one of the technique’s limitations is the failure to detect parasites in individuals with low parasite load. The aim of this study was to establish the prevalence of Schistosoma mansoni in indigenous Maxakali villages, evaluating the TF-Test® performance for diagnosis compared to the Kato-Katz technique. Stool samples from 545 individuals were processed by the TF-Test® (1 sample) and Kato-Katz (1 slide). The positivity rate for S. mansoni by Kato-Katz was 45.7%. The rate by the TF-Test® was 33.2%, and 51.9% by the combined parasitological techniques. The amplitude of parasite load was 24 to 4,056 eggs per gram of feces (epg), with a geometric mean of 139 epg. The co-positivity, co-negativity, and accuracy values by TF-Test® in relation to Kato-Katz were 59.0%, 88.5%, and 75.0%, respectively. The agreement between these techniques was moderate (k=0.486) as determined by the kappa index. Thus, the results of this study demonstrated that the performance of Kato-Katz was superior (p <0.05) to that of TF-Test® in the detection of S. mansoni. The combination of TF-Test® and Kato-Katz resulted in an increased positivity rate of S. mansoni, demonstrating the high risk of infection to which indigenous populations are exposed and the importance of the implementation of control strategies in Maxakali villages.
Keywords: Schistosomiasis, Parasitological techniques, Helminthes, Indigenous populations, Prevalence
INTRODUCTION
Schistosomiasis mansoni continues to be a serious public health problem in Brazil as it is one of the waterborne diseases with a highest prevalence1. The diagnosis of Schistosoma mansoni infection is primarily made through parasitology stool tests, in which the eggs released by the adult female worm are investigated. The Kato-Katz2 technique is recommended by WHO3 for epidemiological studies. However, one of this technique’s limitations is the s failure to detect the parasite in areas of low endemicity and when infected individuals have low parasite load4,5. Due to the low positivity of Kato-Katz in the above-mentioned situation, several authors have proposed new diagnostic methods to increase the diagnosis sensitivity on the field, such as TF-Test® (Three Fecal Test)6, which, in a single step, centrifuges and filters stool samples in formaldehyde-ethyl acetate and concentrates the samples by sedimentation. The rapid diagnosis test proposed by Mello-Silva et al.7, a modified Kato-Katz technique by adding sodium acetate formalin (SAF) as a fixative promotes the clarification of samples to facilitate egg visualization. The FLOTAC technique8, which also uses SAF, is more sensitive for the detection of protozoan and helminthes infections than the Kato-Katz. Therefore, the search for more sensitive diagnostic methods is necessary to obtain a more accurate diagnosis. Applying different parasitology stool tests would be a feasible alternative to overcoming this problem. In addition to parasitological techniques, a diagnosis can be made through immunological and molecular methods, both of which are more sensitive9,10, but are not feasible in epidemiological studies, especially due to the high cost of reagents and equipment. The Point-of-care-circulating Cathodic Antigen (POC-CCA®) has been recently evaluated and is based on the direct detection of the parasite’s CCA antigen in the host’s urine (Rapid Medical Diagnostics, Pretoria, South Africa). Accuracy studies of this test are being conducted to assess its diagnostic performance in areas of low endemicity and in individuals with low parasite burden11.
The Maxakali ethnic group is one of the indigenous communities in the Minas Gerais State that still maintain many of its cultural aspects12. Schistosomiasis affects a large part of this population due to lack of basic sanitation, the presence of intermediate hosts and cultural habits that favor the parasite’s dispersion. Results by Assis et al. 12 point to poor sanitation conditions in these villages, as can be seen by the use of water from rivers and ponds, without any prior treatment, and the absence of an adequate sewage system.
The TF-Test® represents a diagnostic alternative because it is able to detect eggs and larvae of helminthes and protozoan cysts. This test is suitable for individual diagnosis, epidemiological surveys, or chemotherapy evaluation in treated communities6. TF-Test® allows the storage of stool samples for 30 days. Also, the samples can be safely transported when collected in remote areas13. In this study, we compared the performance of this technique for diagnosing schistosomiasis mansoni in Maxakali villages in relation to the Kato-Katz technique, a widelyused parasitological method in epidemiological studies. In this way, we proposed to evaluate the sensitivity of a minimum diagnosis by associating a qualitative method with a quantitative method.
MATERIAL AND METHODS
Study area and population
Indigenous people of the Maxakali ethnic group are distributed in the municipalities of Santa Helena de Minas, Bertopolis, Ladainha and in the district of Topazio in the municipality of Teofilo Otoni, where the following health centers are located: Agua Boa, Pradinho, Aldeia Verde and Cachoerinha, respectively, all of them in the Northeast region of Minas Gerais14. This study was carried out in 2014-2015. The target population included the indigenous population living in the Agua Boa and Pradinho health centers totaling 545 individuals who agreed to participate in the study, 296 (54.3%) female and 249 (45.7%) males.
Inclusion and exclusion criteria
This study included all of the individuals living in the aforementioned villages, of both sexes, aged 0 to 86 years, who agreed to participate in the research and where the leaderships of each village had signed a document authorizing the research. From the 1,642 Maxakali people, 1,026 did not agree to take part of the research/study and 71 failed to successfully finish or failed to accomplish the two diagnostic tests, totaling 545 individuals who agreed to participate in the study.
Diagnostic techniques
Vials for stool specimen collection were distributed to each participant and one sample was returned by each individual.
Kato-Katz – This technique was carried out using the Helmtest® kit (Biomanguinhos, FIOCRUZ, Rio de Janeiro, RJ, Brazil) and following the manufacturer’s instructions. One slide was analyzed per individual, which corresponds to 41.7 mg of feces.
Intensity of infection – The worm burden was determined as eggs per gram of feces (epg) according to WHO3 classification. The value of the individual intensity of infection was obtained according to the Kato-Katz method using the arithmetic mean of eggs found on each slide multiplied by 24, and the population intensity infection was determined by geometric mean2.
TF-Test ® – A portion of feces (about 1 g) was placed in a tube containing preservative solution (10% formalin), which was processed using 3 mL of ethyl acetate and a drop of a neutral detergent, as recommended by the manufacturer (Bio-Brasil Biotecnologia, Anapolis, Goias State, Brazil). This tube was connected to the centrifuge tube and centrifuged at 500 g for 1 min and 30 s in a Laborline – Elektra GoldLine centrifuge. The supernatant was discarded and the total sediment was re-suspended in distilled water and analyzed using optical microscopy (10x e 40x magnification). Three slides containing aliquots of sediment were examined for identification of S. mansoni eggs and analyzed in a qualitative way, i.e., there was no quantification of the parasite’s eggs per gram of feces.
Statistical analysis
OpenEpi software, version 3.03 (www.OpenEpi.com), was used for statistical analysis. Co-positivity, co-negativity, and accuracy values were calculated with 95% confidence intervals (CIs) for the TF-Test® technique. Co-positivity was calculated by the proportion of positive results in both techniques divided by the total number of true positives. Co-negativity was calculated by the proportion of negative results in both techniques divided by the total number of true negatives. Accuracy was calculated by the proportion of true positives and true negatives divided by the total number of evaluated individuals. The gold standard was defined by the sum of positive results for S. mansoni by both parasitological techniques. The level of agreement between different diagnostic techniques was determined by the Kappa coefficient15. The comparison between proportions was calculated by the paired McNemar test and the adopted significance level was 0.05.
Ethical considerations
This study was approved by indigenous leaders, who signed an informed consent form representing all residents in the villages and by the Research Ethics Committee of the Federal University of Ouro Preto (process Nº 2005/58), the National Commission for Research Ethics (CONEP, process Nº 902/2006), and the National Foundation of the Indian (FUNAI; Authorization Nº 73/CGEP/06).
RESULTS
The combined use of Kato-Katz and TF-Test® (the “gold standard”) was able to detect eggs of S. mansoni in 51.9% (283 of 545) of the stool samples. The positivity rate examining only one Kato-Katz slide was 45.7% (249 of 545) and one TF-Test® sample was 33.2% (181/545) (p < 0.05). The co-positivity was 59% (CI: 52.8-64.9%), the co-negativity 88.5% (CI: 84.4-91.7%), and the accuracy, 75% (CI: 71.2-78.5%). The agreement between both diagnostic techniques determined by kappa index was moderate (k=0.486) (Table 1).
Table 1. – Evaluation of performance parameters of the TF-Test® considering Kato-Katz as the “gold standard”.
| Kato-Katz | Co-positivity (%) | Co-negativity (%) | Accuracy (%) | Kappa Index | ||||
|---|---|---|---|---|---|---|---|---|
| Positive | Negative | Total | (CI 95%) | (CI 95%) | (CI 95%) | |||
| TF-Test® | Positive | 147 | 34 | 181 (33.2%)* | 59.0 (52.8-64.9) | 88.5 (84.4-91.7) | 75.0 (71.25 - 78.5) | 0.486 (0.4049-0.5672) |
| Negative | 102 | 262 | 364 | |||||
| Total | 249 (45.7%)* | 296 | 545 | |||||
* McNemar test = 34.0, p<0.001.
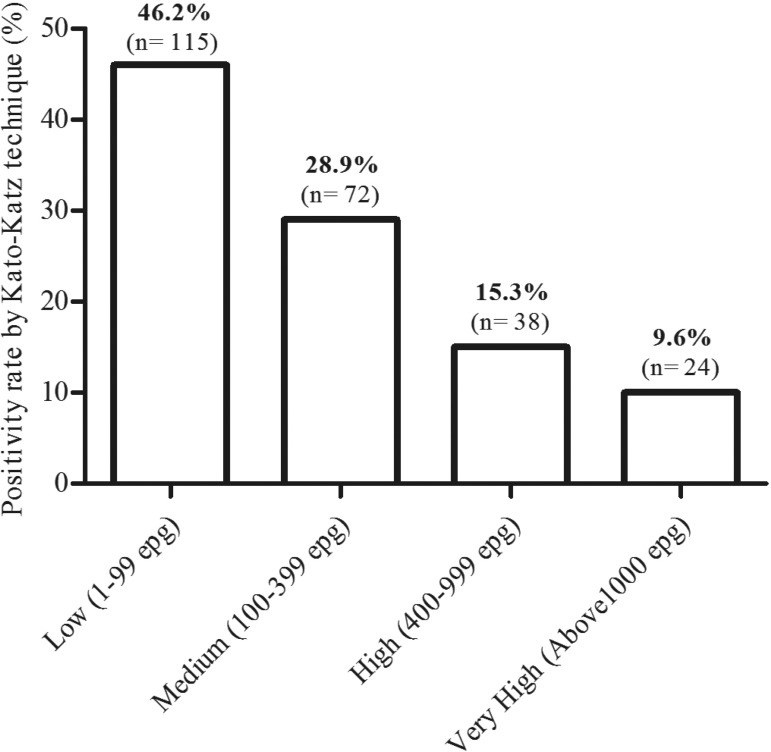
In the Kato-Katz technique, the number of eggs detected per gram of feces varied from 24 to 4,056 epg, with a geometric mean equal to 139 epg, classified as a medium parasite load. The parasite load was low (1 to 99 epg) in 46.2% (115/249) of individuals, medium (100 to 399 epg) in 28.9% (72/249), high (400 to 999 epg) in 15.3% (38/249), and very high (above 1,000 epg) in 9.6% (24/249) (Figure 1).
Figure 1. Relative frequency of positivity by the Kato-Katz technique in relation to worm burden.
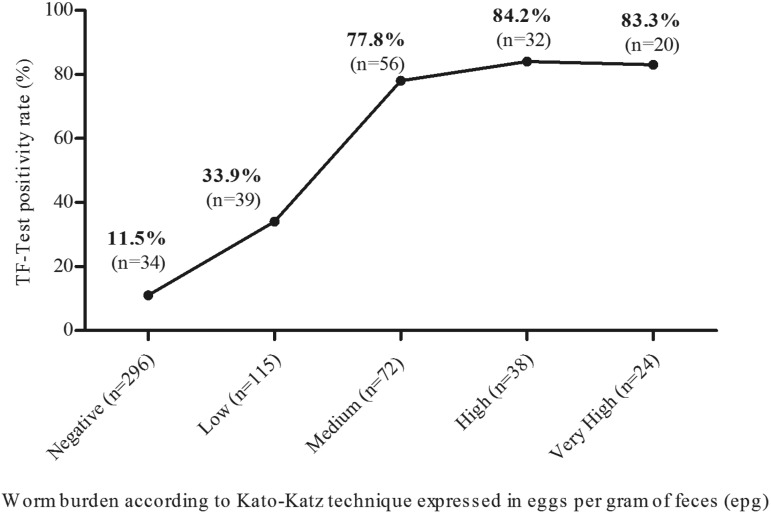
The results presented in Figure 2 have shown that infected individuals with high parasite burden detected by Kato-Katz had a 2.5-fold increase in the positivity rate obtained by the TF-Test, relative to those with low parasite burden. Among those with a negative result by the Kato-Katz technique, 11.5% were detected by the TF-Test®.
Figure 2. Positivity rate for S. mansoni according to the TF-Test® in relation to worm burden obtained by the Kato-Katz technique, Maxakali indigenous people, 2014-2015. Low: 1 – 99 epg; Medium: 100 – 399 epg; High: 400 – 999 epg; Very high: ≥1000 epg. (WHO, 2002).
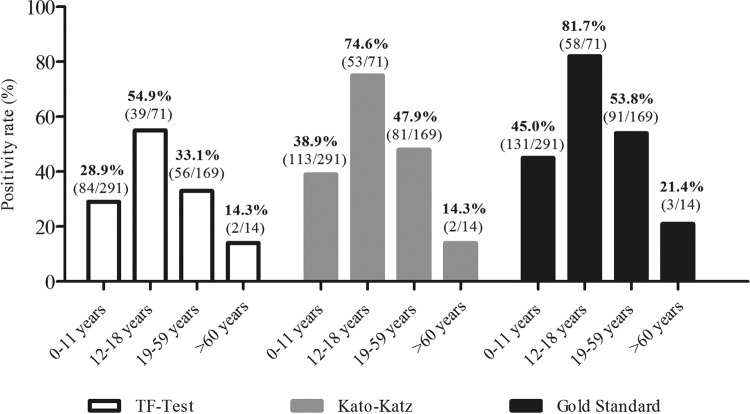
Positivity rates according to the age groups and assessed by the three diagnostic methods are shown in Figure 3. As expected, the “gold standard” results showed the highest positivity rates regardless of the age group, 45% (131/291) in the 0 -11 yearsold group, 81.7% (58/71) in 12-18 group, 53.8% (91/169) in 19-59group , and 21.4% (3/14) in ≥ 60 group. The higher positivity in the 12-18 years old group was followed by a reduction as age increased.
Figure 3. - Positivity rate (%) for schistosomiasis according to age group and diagnostic methods, Maxakali indigenous people, 2014-2015.
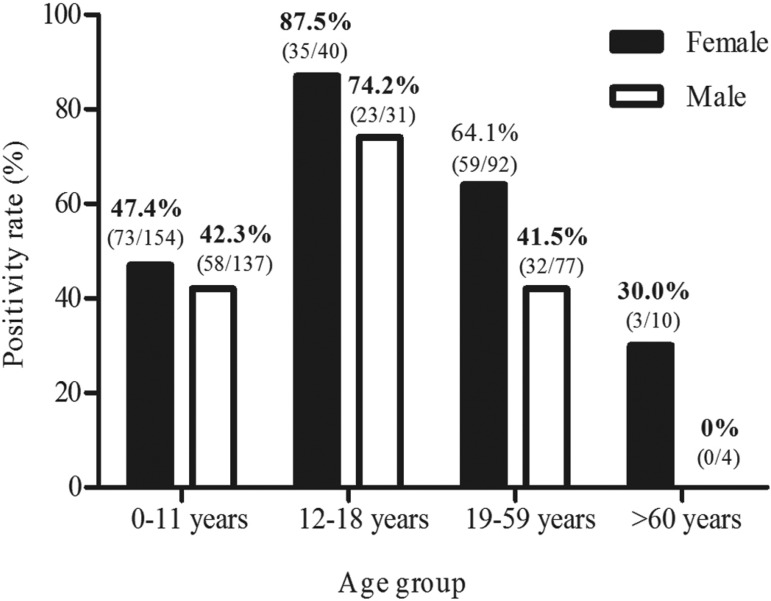
Positivity rates for schistosomiasis according to age group and gender obtained by the “gold standard” are shown in Figure 4. The highest positivity rate (87.5%) was observed among females between 12 and 18 years old. Among the elderly, positivity was observed only in females (30%). The positivity was 57.4% (n=170) in females and 45.4% (n=113) in males. According to the Kato-Katz technique, the female positivity rate was 51% and in males, 39.4%. Using the TF-Test® these rates were 38.9% and 26.5% for males and females, respectively.
Figure 4. - Positivity rate for schistosomiasis according to age group and gender obtained by the Gold Standard*. *Gold Standard: Results obtained by the combination of Kato-Katz and TF-Test® techniques.
DISCUSSION
Diagnosis of S. mansoni infection has traditionally been carried out by parasitological methods such as the Kato-Katz technique2, which provides good sensitivity in highly endemic areas and has been widely applied in epidemiological surveys due to its favorable cost-benefit and practicality in precarious laboratory infrastructure situations, since it is a quantitative technique and allows the concomitant diagnosis of other helminths4,16. However, in low prevalence situations, in individuals presenting low infection intensity, as well as in the evaluation of cure after specific treatment, this technique has been less sensitive4,5,10.
The innovative proposal of the TF-Test® technique is based on the centrifugal sedimentation of feces in formaldehyde-ethyl acetate. The differential of this technique in relation to others is its high diagnostic sensitivity for helminthes and protozoa6. This could be attributed to the technique affording high parasitic concentration, when processing three fecal samples collected on different days, in a single step. In this study, only one fecal sample per individual was used to conduct a comparative evaluation between methods, aimed at greater compliance by the participants and reducing logistic problems in carrying out the study. Moreover, the one fecal sample procedure was designed to evaluate the effectiveness of TF-Test® associated with Kato-Katz to estimate the schistosomiasis mansoni prevalence in vulnerable populations.
The use of a single fecal sample to perform the TF-Test® can be justified by the logistic limitations to carry out sampling on alternate days in the field work, as recommended by the manufacturer. A previous study in the same ethnic group has reported inadvertent ingestion of the formaldehyde from TF-Test® tubes by some study participants12. For this reason, one single sample was used to evaluate the effectiveness of TF-Test® in the present study. The sample was collected in regular plastic containers and transferred to the TF-Test® tube by the health care team, on the same day. A decrease in sensitivity to detect parasite infection was expected when using one fecal sample compared to multiple samples. However, the combination of two methods is expected to compensate this lack of sensitivity. These results demonstrated that this strategy is feasible in indigenous villages presenting the same problem and exposed to a high risk of infection with intestinal parasites.
The TF-Test® technique presented a lower positivity rate for S. mansoni (33.2%) than that obtained by a slide analyzed by the Kato-Katz technique (45.7%), taking into account the sensitivity of only 59% and a moderate agreement (kappa index: 0.486) in relation to the Kato-Katz technique. Our data corroborate the findings of Siqueira et al.5 and differ from those obtained by Gomes et al.6 and Carvalho et al.13, who found that the TF-Test® had higher positivity rates in detecting both protozoa and helminthes in human stool samples as compared with the conventional Kato-Katz. These results could be explained by the recommended use of three stool samples from each subject collected on alternate days followed by these authors, whereas in this study, a single fecal sample per individual was used to perform the test. According to Assis et al.12, the prevalence of S. mansoni determined by the TF-Test®, using three fecal samples on alternating days, was 23.7% for the entire Maxakali Indigenous Land, 17.6% for the Agua Boa Base Center, and 23.9% for the Pradinho Base Center, both lower than the 33.2% obtained in this study. Some factors may justify the positivity rate obtained by the TF-Test® technique in these areas. Although the amount of fecal material used by this technique is greater, parasitic forms are probably lost during processing17 and also because fecal debris causes the slide to be dirtier, making it difficult to visualize the parasite’s eggs.
The parasite load presented by infected individuals was moderate and quantified by the Kato-Katz technique only. Although the quantification of infection intensity has long been considered an important factor in the evaluation of clinical morbidity, recent studies have pointed out that the severity of the disease does not correlate only with the worm burden, as in neuroschistosomiasis and myelorradiculopathy18,19. The epidemiological relevance of the infection intensity is due to the fact that it reflects the individual’s potential for spreading the disease and contributing to its transmission20. One of the limitations of the TF-Test® is that it is a qualitative technique; therefore, it does not allow the infection quantification. However, in some studies, quantification has been experimentally performed, as in the study by Lumina et al. 21, in which the TF-Test® was used to diagnose intestinal parasites in sheep.
It was possible to clearly observe that the positivity rate obtained by the TF-Test® technique is directly influenced by the individual’s parasite load, with rates ranging from 33.9% in those individuals with a low parasite load to 84.2% in those with a high load. This is a significant increase of 2.5 times, proving that the TF-Test® presented a lower than expected diagnostic performance, since it uses a larger amount of fecal material, which theoretically would lead to an increase in efficacy. It is important to emphasize that the TF-Test® detected 11.5% positivity in individuals who presented Kato-Katz negative results, thus demonstrating the importance of the association of diagnostic methods for the better detection of infected individuals.
Although the TF-Test® has been considered poorly effective in detecting S. mansoni as compared to Kato-Katz, it was more sensitive to detect other intestinal parasites. This test found 46.8% of positivity for hookworm versus 22.8% with Kato-Katz; 13.8% for Hymenolepis nana versus 0.2% with Kato-Katz; 13.0% for Strongyloides stercoralis, and 28.3% for pathogenic protozoan cysts. The ability to detect helminthes larvae and protozoan cysts is a positive characteristic of TF-Test® in relation to Kato-Katz. The high prevalence of non-pathogenic protozoa, such as Entamoeba coli (74.5%), Endolimax nana (56.7%), and Iodamoeba butschlii (29.9%), indicates the precarious situation of the Maxakali people with regard to sanitary conditions, hygiene and health, since these organisms indicate fecal contamination22 and their presence is considered an indicator of the poorquality of drinking water.
The highest rates for S. mansoni infection were obtained in the 12-18 age group regardless of the diagnostic technique, followed by the 19-59 and 0-11 age groups, and then decreasing with age. The highest positivity rates obtained in children and young people were probably due to a higher exposure and contact with contaminated water, associated with occupational, leisure, or household activities. According to Enk et al.4, males in the age group between 10 and 30 years old and practicing leisure activities related to contact with unsafe water show the highest accumulative risk of infection. Our data differ from those reported by these authors, since the highest positivity rate was found in females ranging from 12-18 years old.
In general, the probable source of infection could be attributed to precarious sanitary conditions: 73.8% used water from rivers and ponds, 98.7% used untreated water, and 74.7% did not have toilets12. Another fact would be their behavior in relation to water, as they people collectively bathe, wash clothes, and draw water from the same sources, and both men and women use a fishing basket in the water. Moreover, the semi-nomadic habits of the Maxakali people and frequent familial mobility to and from endemic regions in the Mucuri and Doce River Valleys, could also be considered as probable sources of infection12.
Other aspects should be taken into account when applying the TF-Test® in epidemiological studies, such as the need to acquire kits and inputs to carry out the test, along with a centrifuge to process the samples. In spite of the Kato-Katz technique’s advantages, such as cost-benefit, simplicity, and infrastructure, it presents similar limitations of other parasitological tests, such as the need for personnel capable of microscopic reading, which is a laborious step that requires a well-trained examiner to avoid false-positive and/or false-negative results, in addition to detecting helminthes eggs only. In contrast, the TF-Test® detects a broader spectrum of parasites.
It should be emphasized that determining the prevalence of schistosomiasis is directly influenced by the disease’s transmission force in a given area. In areas of low endemicity23, the number of slides and samples examined by the Kato-Katz technique needs to be increased4,5,10. It was possible to detect a large number of infected individuals in this study by examining a single slide of Kato-Katz because it was carried out in an area with high endemicity.
One of the limitations of this study was the participants’ low level of compliance, which can be attributed to cultural aspects inherent to this indigenous population, as well as its unfamiliarity with access to health services.
It is widely reported in the literature that the combination of diagnostic techniques or increased numbers of samples and slides made by the Kato-Katz technique increases the detection of disease from 2.5 to 4.5 times4,5. In conclusion, despite the limiting factors intrinsic to each technique, the combined used of the TF-Test® technique with Kato-Katz resulted in an increase in the positivity rate of S. mansoni detection from 45.7% to 51.9% (p <0.05), demonstrating the importance of the association of diagnostic methods for a better detection of infected individuals. The combination of a quantitative and a qualitative method performed with only one fecal sample collection was effective in classifying the Maxakali population as a high risk group for schistosomiasis infection. The Maxakali are in socially vulnerable conditions12 and, as such, the results of this study are expected to act as indicators of the prevalence of schistosomiasis and assist in targeting control strategies such as basic sanitation, health education, and treatment, which need to be deployed in this area.
ACKNOWLEDGMENTS
We would like to acknowledge the indigenous leadership, the Distrito Sanitario Especial Indigena (DSEI-MG/ES), Secretaria Especial de Saude Indigena (SESAI), to the indigenous people of the Maxakali ethnic group, to the health agents and to the technical laboratories that collaborated to carry out the diagnostic tests.
Footnotes
FINANCIAL SUPPORT
This work was supported by the Federal University of Ouro Preto (UFOP); the National Council for Scientific and Technological Development (CNPq), grant Nº 305999/2014-1; and the Foundation for Research Support in Minas Gerais (FAPEMIG), grant Nº PPM-00154-12.
REFERENCES
- 1.Brasil. Ministério da Saúde. Secretaria de Vigilância em Saúde. Departamento de Vigilância das Doenças Transmissíveis . Vigilância da esquistossomose mansoni: diretrizes técnicas. 4ª. Brasília: Ministério da Saúde; 2014. [Google Scholar]
- 2.Katz N, Chaves A, Pellegrino J. A simple device for quantitative stool thick-smear technique in schistosomiasis mansoni. Rev Inst Med Trop Sao Paulo. 1972;14:397–400. [PubMed] [Google Scholar]
- 3.World Health Organization . Prevention and control of schistosomiasis and soil-transmitted helminthiasis: report of a WHO expert committee. Geneva: WHO; 2002. [PubMed] [Google Scholar]
- 4.Enk MJ, Lima AC, Drummond SC, Schall VT, Coelho PM. The effect of the number of stool samples on the observed prevalence and the infection intensity with Schistosoma mansoni among a population in an area of low transmission. Acta Trop. 2008;108:222–228. doi: 10.1016/j.actatropica.2008.09.016. [DOI] [PubMed] [Google Scholar]
- 5.Siqueira LM, Coelho PM, Oliveira AA, Massara CL, Carneiro NF, Lima AC, et al. Evaluation of two coproscopic techniques for the diagnosis of schistosomiasis in a low transmission area in the state of Minas Gerais, Brazil. Mem Inst Oswaldo Cruz. 2011;106:844–850. doi: 10.1590/s0074-02762011000700010. [DOI] [PubMed] [Google Scholar]
- 6.Gomes JF, Hoshino-Shimizu S, Dias LC, Araújo AJ, Castilho VL, Neves FA. Evaluation of a novel kit (TF-Test®) for the diagnosis of intestinal parasitic infections. J Clin Lab Anal. 2004;18:132–138. doi: 10.1002/jcla.20011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Mello-Silva CC, João RC, Augusto RC, Santos CP. A rapid diagnostic test for schistosomiasis mansoni. Mem Inst Oswaldo Cruz. 2013;108:1078–1080. doi: 10.1590/0074-0276130335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Knopp S, Speich B, Hattendorf J, Rinaldi L, Mohammed KA, Khamis IS, et al. Diagnostic accuracy of Kato-Katz and FLOTAC for assessing anthelmintic drug efficacy. PLoS Negl Trop Dis. 2011;5:e1036. doi: 10.1371/journal.pntd.0001036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Cavalcanti MG, Silva LF, Peralta RH, Barreto MG, Peralta JM. Schistosomiasis in areas of low endemicity: a new era in diagnosis. Trends Parasitol. 2013;29:75–82. doi: 10.1016/j.pt.2012.11.003. [DOI] [PubMed] [Google Scholar]
- 10.Siqueira LM, Gomes LI, Oliveira E, Oliveira ER, Oliveira AA, Enk MJ, et al. Evaluation of parasitological and molecular techniques for the diagnosis and assessment of cure of schistosomiasis mansoni in a low transmission area. Mem Inst Oswaldo Cruz. 2015;110:209–214. doi: 10.1590/0074-02760140375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Siqueira LM, Couto FF, Taboada D, Oliveira ÁA, Carneiro NF, Oliveira E, et al. Performance of POC-CCA® in diagnosis of schistosomiasis mansoni in individuals with low parasite burden. Rev Soc Bras Med Trop. 2016;49:341–347. doi: 10.1590/0037-8682-0070-2016. [DOI] [PubMed] [Google Scholar]
- 12.Assis EM, Olivieria RC, Moreira LE, Pena JL, Rodrigues LC, Machado-Coelho GL. Prevalência de parasitos intestinais na comunidade indígena Maxakali, Minas Gerais, Brasil, 2009. Cad Saude Publica. 2013;29:681–690. doi: 10.1590/s0102-311x2013000800006. [DOI] [PubMed] [Google Scholar]
- 13.Carvalho GL, Moreira LE, Pena JL, Marinho CC, Bahia MT, Machado-Coelho GLL. A comparative study of the TF-Test®, Kato-Katz, Hoffman-Pons-Janer, Willis and Baermann-Moraes coprologic methods for the detection of human parasitosis. Mem Inst Oswaldo Cruz. 2012;107:80–84. doi: 10.1590/s0074-02762012000100011. [DOI] [PubMed] [Google Scholar]
- 14.Minas Gerais. Governo do Estado. Secretaria de Planejamento e Gestão . Marco de referência: povos indígenas em Minas Gerais. Belo Horizonte: Secretaria de Planejamento e Gestão; 2009. [Google Scholar]
- 15.Landis JR, Koch GG. The measurement of observer agreement for categorical data. Biometrics. 1977;33:159–174. [PubMed] [Google Scholar]
- 16.Gomes LI, Enk MJ, Rabello A. Diagnosing schistosomiasis: where are we? Rev Soc Bras Med Trop. 2014;47:3–11. doi: 10.1590/0037-8682-0231-2013. [DOI] [PubMed] [Google Scholar]
- 17.Mendes CR, Teixeira AT, Pereira RA, Dias LC. Estudo comparativo de técnicas parasitológicas: Kato-Katz e coprotest® . Rev Soc Bras Med Trop. 2005;38:178–180. doi: 10.1590/s0037-86822005000200010. [DOI] [PubMed] [Google Scholar]
- 18.Lambertucci JR, Silva LC, do Amaral RS. Guidelines for the diagnosis and treatment of schistosomal myeloradiculopathy. Rev Soc Bras Med Trop. 2007;40:574–581. doi: 10.1590/s0037-86822007000500016. [DOI] [PubMed] [Google Scholar]
- 19.Vale TC, Sousa-Pereira SR, Ribas JG, Lambertucci JR. Neuroschistosomiasis mansoni: literature review and guidelines. Neurologist. 2012;18:333–342. doi: 10.1097/NRL.0b013e3182704d1e. [DOI] [PubMed] [Google Scholar]
- 20.Araújo AJ, Kanamura HY, Dias LC, Gomes JF, Araújo SM. Coprotest® quantitativo: quantificação de ovos de helmintos em amostras fecais utilizando-se sistema de diagnóstico comercial. J Bras Patol Med Lab. 2003;39:115–124. [Google Scholar]
- 21.Lumina G, Bricarello PA, Gomes JF, Amarante AF. Avaliação do kit “TF-Test” para o diagnóstico das infecções por parasitas gastrintestinais em ovinos. Braz J Vet Res Anim Sci. 2006;43:496–501. [Google Scholar]
- 22.Rocha SR, Silva JG, Peixoto SV, Caldeira RL, Firmo JO, Carvalho OS, et al. Avaliação da esquistossomose e de outras parasitoses intestinais, em escolares do município de Bambuí, Minas Gerais, Brasil. Rev Soc Bras Med Trop. 2000;33:431–436. [PubMed] [Google Scholar]
- 23.Alarcón de Noya B, Ruiz-Guevara R, Colmenares C, Losada S, Contreras R, Noya O. Low transmission areas of schistosomiasis in Venezuela: consequences on the diagnosis, treatment, and control. Mem Inst. Oswaldo Cruz. 2006;101(Suppl 1):29–35. doi: 10.1590/s0074-02762006000900006. [DOI] [PubMed] [Google Scholar]