Table 1.
Selected examples of the O-cyanation/hetero oxy-Cope rearrangement.a
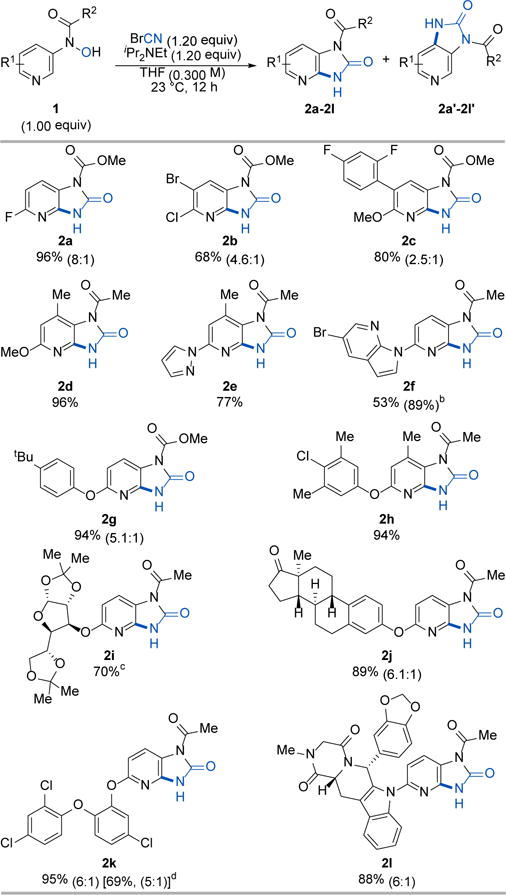
|
Cited yields and isomeric ratios are of isolated material by column chromatography.
Yield in parenthesis was determined by 1H NMR analysis of the crude reaction mixture with CH2Br2 an internal standard.
The reaction exhibited 6.4:1 selectivity, but the minor product came with inseparable impurities. The yield reported here is of the major isomer only.
Yield in the bracket is the isolated yield of a gram-scale reaction.
