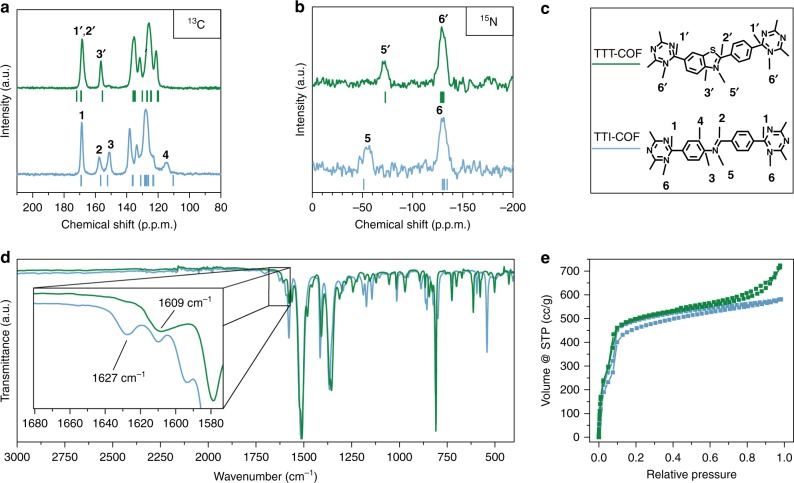
Fig. 2.
Characterization of the TTI-COF (blue) and TTT-COF (green). a 13C ssNMR demonstrating the conversion of the imine linkage to the corresponding thiazole. b 15N ssNMR showing a shift in the imine nitrogen position (5 → 5′). Calculated Δδ values for the TTT and TTI-COF on B97-2/pcS-2 level of theory are shown as red and black dashes, respectively. c Assignment of the 13C and the 15N ssNMR signals to the respective 13C and 15N nuclei in the structures. d FT-IR spectra of TTI-COF (black) and TTT-COF (red). The inset shows an enlargement of the region characteristic for N = C vibrations. e Argon sorption isotherms of TTI-COF and TTT-COF showing retention of porosity