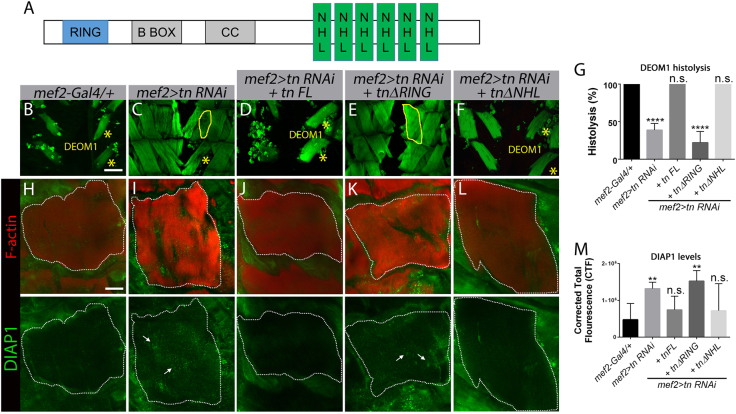
Fig. 6. The RING domain is required for DEOM histolysis and to maintain DIAP1 protein levels.
a Schematic diagram showing the conserved RING and NHL domains in Tn. The B-box and coiled-coil domains are predicted, but poorly conserved. b–f Confocal Z-stack micrographs of abdominal muscles at 24 h APF stained for F-actin (green). b DEOM1 underwent histolysis (*) in the mef2-Gal4/+ control genotype. c DEOM1 histolysis is incomplete (yellow solid line) in mef2>tn RNAi animals. d–f Expression of Tn FL (d) or TnΔNHL (f) restores histolysis, while removal of the RING domain completely blocks DEOM degeneration, indicating this region is essential for normal histolysis (e). g Bar graph showing a significant decrease in DEOM histolysis in mef2>tn RNAi; tnΔRING flies. h–l Confocal Z-stack merged images of DEOM1 (white dotted line) at 12 h APF co-stained for F-actin (red) and DIAP1 (green). Low DIAP1 levels are observed in genotypes in which DEOM histolysis proceeds normally, including mef2-Gal4 (h), mef2>tn RNAi+tn FL (j), and mef2>tn RNAi+tnΔNHL (l). Increased DIAP1 levels are observed in mef2>tn RNAi (i) or mef2>tn RNAi+tnΔRING (k) DEOMs. m Quantification of relative fluorescence intensity levels reveals significantly higher DIAP1 levels in mef2>tn RNAi+tnΔRING muscles. Mean ± SEM (n.s., not significant, ****p < 0.001, **p < 0.01). Scale bar, 100 µm (b–f) and 50 µm (h–l)