Abstract
Cellulose and lignin are the main polymeric components of the forest litter horizon. We monitored microbial community composition using phospholipid fatty acid (PLFA) analysis and investigated the ligninolytic and cellulolytic enzyme activities of the litter horizon across an alpine treeline ecotone in the eastern Tibetan Plateau. The activities of ligninolytic and cellulolytic enzymes and the biomass of microbial PLFAs were higher in the initial stage of litter decomposition than in the latter stage in the three vegetation types (coniferous forest, alpine shrubland and alpine meadow). Soil microbial community structure varied significantly over the course of litter decomposition in the three vegetation types. Furthermore, the BIOENV procedure revealed that the carbon to nitrogen (C:N) ratio, carbon to phosphorus (C:P) ratio and moisture content (MC) were the most important determinants of microbial community structure in the initial stage of litter decomposition, whereas pH and the lignin concentration were the major factors influencing the microbial community structure in the later stage of litter decomposition. These findings indicate that litter quality drives the differentiation of microbial communities in the litter horizon across an alpine treeline ecotone in the eastern Tibetan Plateau.
Introduction
The decomposition of plant litter involves a complex set of processes that include chemical, physical, and biological agents acting upon a wide variety of organic substrates. Based on the degree of decomposition, the litter horizon can typically be divided into a fresh litter (L) layer, a fermentation (F) layer and a humus (H) layer1–3. These three layers largely represent different degrees of decomposition of organic matter. The L layer is the upper layer and is formed by recognizable plant and soil animal remains. Below this layer is a layer that typically consists of a mixture of organic matter in different stages of decomposition, known as the F layer. The third layer is the H layer, which largely consists of humified material with little or no visible plant structure1,2. However, in some cases, the F layer is thin or absent and difficult to distinguish from the L layer. Therefore, the litter horizon can also be divided into a fresh litter and fermentation (LF) layer and a humus (H) layer4, which represent the initial and later stages of litter decomposition, respectively4,5. The LF layer is the upper 2 cm layer that contains easily recognizable plant remains that show some discoloration4,6. The 2 to 5 cm layer comprises humified material without recognizable plant structures except for stems and coarse root remains, and it is referred to as the H layer4. Some nutrients and low-molecular-weight compounds, notably sugars, are readily lost from the litter through dissolution and leaching in the initial stages of litter decomposition and due to the action of rapidly growing microorganisms. In contrast, larger macromolecules, such as cellulose, hemicelluloses, and lignin, are degraded more slowly in the later stages of litter decomposition7. During soil transformation, the litter horizon is formed from both lignocellulose and the structural components of microbial decomposers8. Moreover, the degradation of lignocellulose is a critical component of the decomposition of the fraction of global carbon (C) in the soil, which is the largest C pool on Earth.
Microorganisms, such as white rot, brown rot, and soft rot, are the primary decomposers in forest soil systems and the main producers of enzymes that decompose lignin and cellulose1,9, the two most abundant phytochemicals in soils. Thus, microorganisms are the most important players in litter decomposition10,11. The microbial products of decomposition become the main precursors of stable soil organic matter by promoting aggregation and by forming strong chemical bonds with the mineral soil matrix12. Ligninases and cellulases are important extracellular C-acquiring enzymes involved in litter decomposition. The lignin decomposition system consists of three principle ligninases: laccase, manganese peroxidase (MnP) and lignin peroxidase (LiP)13. Cellulose-degrading microorganisms secrete numerous cellulolytic enzymes (mainly cellobiohydrolase, endo-1,4-β-D-glucanase (EG) and β-glucosidase (BG)), which act synergistically to completely degrade lignocellulosic biomass14,15.
According to previous studies, litter substrate quality varies with the stage of litter decomposition1,16. However, little is known regarding the determinants of microbial communities and the differentiation of the relationships between litter chemical properties and the structure of the microbial community in the different stages of litter decomposition. The Microbial Efficiency-Matrix Stabilization (MEMS) framework suggests that labile plant constituents are utilized more efficiently by microbes during the initial stages of litter decomposition12. Due to the differences among the different stages of litter decomposition in chemical properties and available carbon for microorganisms, we hypothesized that lignocellulolytic enzyme activity and microbial community structure are constantly changing during the different stages of litter decomposition.
The alpine treeline ecotone is the zone that extends from closed subalpine forest (timberline) to the upper boundary of tree distribution. This ecotone does not typically occur as an abrupt line; rather, it typically appears as a patchy transition that consists of several intermediate vegetation zones and components, such as dark coniferous forest, alpine shrubland and alpine meadow17. An alpine climate and low land-surface temperatures (with an annual average temperature of 6 to 12 °C) result in a low rate of litter decomposition in the alpine treeline ecotone. Previously, we studied the litter decomposition rate and the release of carbon and nutrients during litter decomposition17 and the mass loss and lignocellulolytic enzyme activities of residues and leaf litter in a coniferous forest and timberline5. However, we did not investigate microbial community structure during litter decomposition. Therefore, in the present study, we monitored microbial community composition using phospholipid fatty acid (PLFA) analysis and assessed the ligninolytic and cellulolytic enzyme activities of the litter horizon across an alpine treeline ecotone in the eastern Tibetan Plateau. Our objectives were to answer two questions: (1) How do lignocellulolytic enzyme activities, microbial PLFA biomasses and community structure differ between the two litter decomposition stages for the three vegetation types? (2) How do the relationships between litter physicochemical properties (e.g., soil organic carbon (SOC), total nitrogen (TN) and total phosphorus (TP)) and microbial community structure differ between the two litter decomposition stages?
Results
Litter physicochemical properties
The SOC, lignin and cellulose concentrations and the C:N, C:P, and N:P ratios of the LF layer were higher than those of the H layer for all three vegetation types (coniferous forest, alpine shrubland and alpine meadow) (all p < 0.05, Table 1), and TP was lower in the LF layer than in the H layer for all three vegetation types (all p < 0.05, Table 1). There was no significant difference in TN between the LF and H layers for any of the three vegetation types (Table 1). Moisture content and pH were lower in the LF layer than in the H layer in alpine meadow (p < 0.05, Table 1). pH was higher in the LF layer than in the H layer in coniferous forest (p < 0.05, Table 1).
Table 1.
Chemical properties of the litter layers.
| Variable | Coniferous forest | Alpine shrubland | Alpine meadow | |||
|---|---|---|---|---|---|---|
| LF layer | H layer | LF layer | H layer | LF layer | H layer | |
| SOC (g kg–1) | 330 ± 40.9a | 201.8 ± 51.5b | 284.5 ± 70.1a | 210.2 ± 81.7b | 415.7 ± 42.3a | 115.4 ± 26.0b |
| TN (g kg–1) | 9.4 ± 1.3a | 8.5 ± 1.0a | 9.4 ± 2.1a | 8.8 ± 0.8a | 7.4 ± 0.7a | 7.1 ± 1.3a |
| TP (g kg–1) | 2.3 ± 0.6b | 2.9 ± 0.5a | 2.1 ± 0.5b | 2.4 ± 0.4a | 1.9 ± 0.7b | 2.4 ± 0.3a |
| C:N | 35.5 ± 5.4a | 23.5 ± 4.5b | 30.4 ± 4.9a | 23.7 ± 8.5b | 57.0 ± 8.9a | 16.4 ± 2.5b |
| C:P | 152.8 ± 35a | 72.6 ± 22.8b | 141.5 ± 38a | 86.8 ± 31.5b | 245.8 ± 76.7a | 49 ± 11.4b |
| N:P | 4.3 ± 0.8a | 3.1 ± 0.7b | 4.7 ± 1.4a | 3.7 ± 0.5b | 4.4 ± 1.6a | 3.0 ± 0.5b |
| MC (%) | 54.0 ± 15.2a | 48.9 ± 13.3a | 60 ± 8.3a | 60.4 ± 5a | 16.4 ± 6.7b | 46.2 ± 6.3a |
| pH | 5.5 ± 0.2a | 4.8 ± 0.2b | 5.7 ± 0.2a | 5.5 ± 0.3a | 5.7 ± 0.1b | 6.0 ± 0.1a |
| Cellulose (%) | 34.8 ± 5.9a | 16.3 ± 6.3b | 28.5 ± 11.4a | 16.1 ± 6b | 37.0 ± 4.6a | 7.9 ± 3.1b |
| Lignin (%) | 25.5 ± 3.5a | 14.8 ± 6.1b | 17.1 ± 6.4a | 14.3 ± 3.4a | 12.2 ± 5a | 6.2 ± 2.9b |
The results are the means ± standard errors of 15 replicates. Lowercase letters indicate significant differences (p < 0.05) between different layers within the same vegetation type as identified by the Mann-Whitney U test. SOC, soil organic carbon; TN, total nitrogen; TP, total phosphorus; MC, moisture content.
Lignocellulolytic enzyme activity
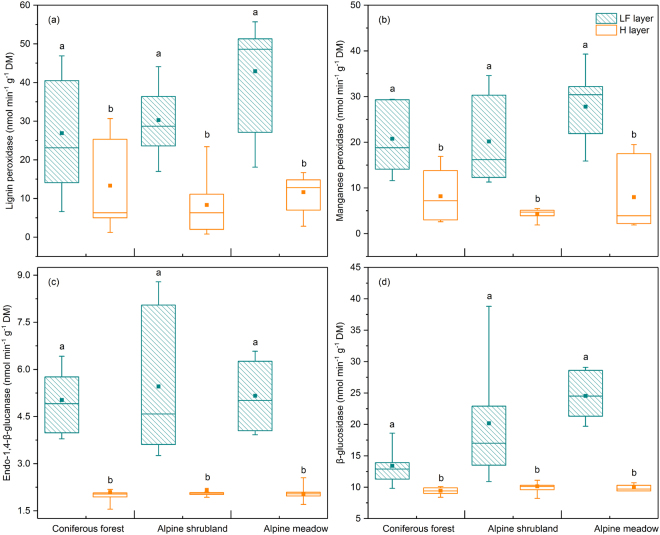
The activities of LiP, MnP, EG, and BG were significantly higher in the LF layer than in the H layer in all three vegetation types (all p < 0.05, Fig. 1). The Pearson correlation analysis showed that the activities of LiP, MnP, EG, and BG were positively correlated with SOC; the C:N, C:P and N:P ratios; and cellulose concentrations (all p < 0.01, Table 2). The activities of LiP, MnP, EG, and BG were negatively correlated with TP and MC (except MnP with TP, p < 0.05, and EG with MC, p = 0.206; all p < 0.01, Table 2).
Figure 1.
Enzymatic activities in the LF and H layers in coniferous forest, alpine shrubland and alpine meadow. The boxes represent the range of the first and third quartiles; the horizontal lines in the boxes represent the median; points within each boxplot represent the means; the upper and lower bounds of the bars reflect the 90th and 10th percentiles, respectively. Bars with different lowercase letters indicate significant differences (p < 0.05) between the LF and H layers within the same vegetation type as identified by the Mann-Whitney U test.
Table 2.
Pearson correlation coefficients of enzyme activities and chemical properties (n = 90).
| Enzyme | SOC | TN | TP | C:N | C:P | N:P | MC | pH | Cel | Lig |
|---|---|---|---|---|---|---|---|---|---|---|
| LiP | 0.577** | −0.040 | −0.420** | 0.627** | 0.682** | 0.425** | −0.450** | 0.135 | 0.581** | 0.091 |
| MnP | 0.663** | 0.116 | −0.265* | 0.632** | 0.639** | 0.371** | −0.372** | 0.147 | 0.673** | 0.242* |
| EG | 0.617** | 0.165 | −0.330** | 0.556** | 0.608** | 0.475** | −0.135 | 0.143 | 0.676** | 0.340** |
| BG | 0.617** | 0.071 | −0.436** | 0.624** | 0.691** | 0.499** | −0.457** | 0.250* | 0.536** | 0.032 |
Values are Pearson correlation coefficients. “*” and “**” indicate significance at the 0.05 and 0.01 levels, respectively. Enzyme abbreviations are defined in Table 5. SOC, soil organic carbon; TN, total nitrogen; TP, total phosphorus; MC, moisture content; Cel, cellulose concentration; Lig, lignin concentration
The biomass of microbial PLFAs and microbial community structure
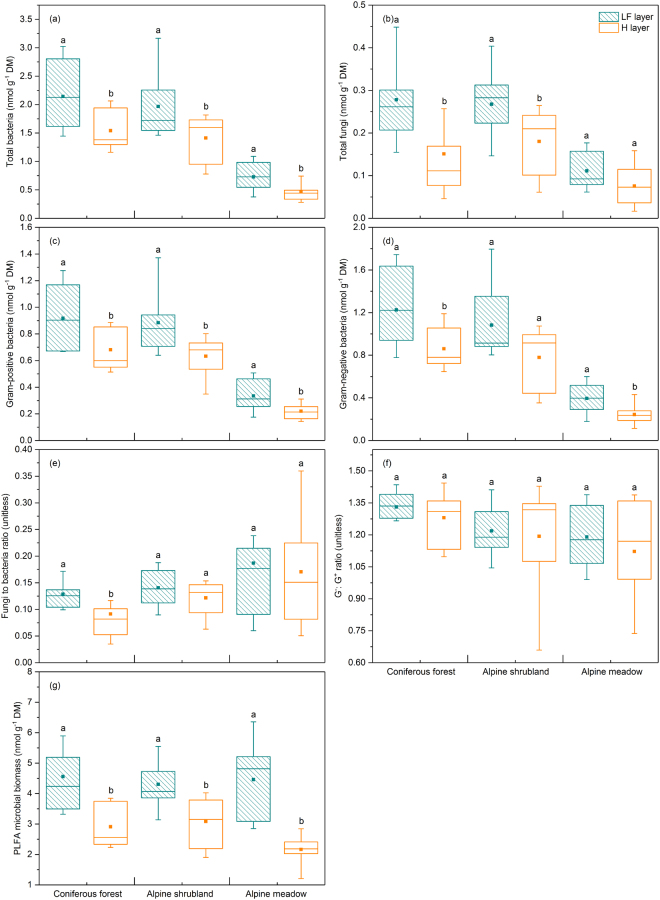
The PLFA contents of TB (total bacteria), TF (total fungi), G+ (gram-positive bacteria), G− (gram-negative bacteria) and MB (microbial biomass) of the LF layer were significantly higher than those of the H layer in coniferous forest (all p < 0.05, Fig. 2). The PLFA contents of TB, TF, G+ and MB were significantly higher in the LF layer than in the H layer in alpine shrubland (all p < 0.05, Fig. 2). The PLFA contents of TB, G+, G− and MB were markedly higher in the LF layer than in the H layer in alpine meadow (all p < 0.05, Fig. 2). For the ratio of fungi to bacteria (F:B), a significant difference between the LF and H layers was observed only in coniferous forest (p < 0.05, Fig. 2e). No significant difference in the ratio of gram-negative to gram-positive bacteria (G−:G+) between the LF and H layers was observed for any of the three vegetation types (coniferous forest, alpine shrubland and alpine meadow) (Fig. 2f).
Figure 2.
The concentrations of microbial PLFAs and ratios of biomarkers in the LF and H layers in coniferous forest, alpine shrubland and alpine meadow. The boxes represent the range of the first and third quartiles; the horizontal lines in the boxes represent the median; points within each boxplot represent the means; the upper and lower bounds of the bars reflect the 90th and 10th percentiles, respectively. Bars with different lowercase letters indicate significant differences (p < 0.05) between the LF and H layers within the same vegetation type as identified by the Mann-Whitney U test. G−:G+, ratio of gram-negative bacteria to gram-positive bacteria.
The non-metric multidimensional scaling (NMDS) analysis clearly showed variation in the microbial community between the LF and H layers in all three vegetation types (stress = 0.065, Fig. 3). The significance of the patterns observed in NMDS was confirmed by PERMANOVA (all p < 0.01, Table 3).
Figure 3.
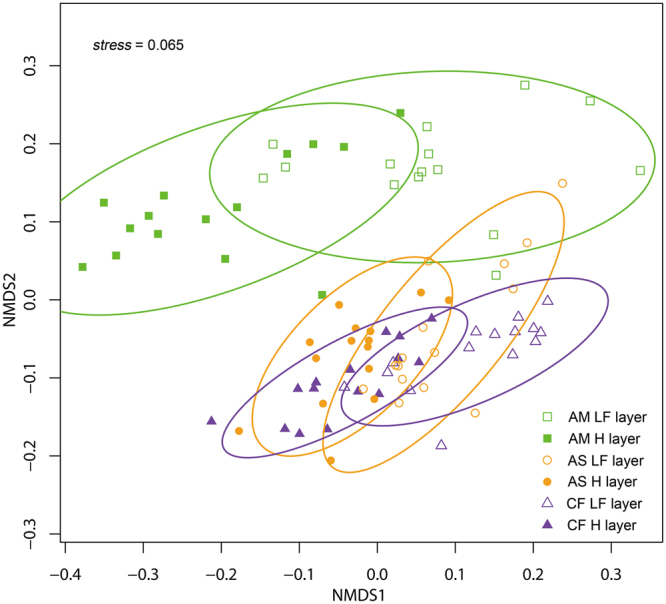
Bray-Curtis-based NMDS of the PLFAs in the LF and H layers in coniferous forest (CF), alpine shrubland (AS) and alpine meadow (AM).
Table 3.
Results from the PERMANOVA. Microbial community Bray-Curtis dissimilarity was modeled as response variable.
| Vegetation type | pair | F | R 2 | p |
|---|---|---|---|---|
| Coniferous forest | LF layer vs. H layer | 20.709 | 0.425 | 0.001 |
| Alpine shrubland | LF layer vs H layer | 9.507 | 0.253 | 0.001 |
| Alpine meadow | LF layer vs H layer | 19.111 | 0.406 | 0.001 |
Correlations between microbial community structure, litter physicochemical properties and enzyme activities
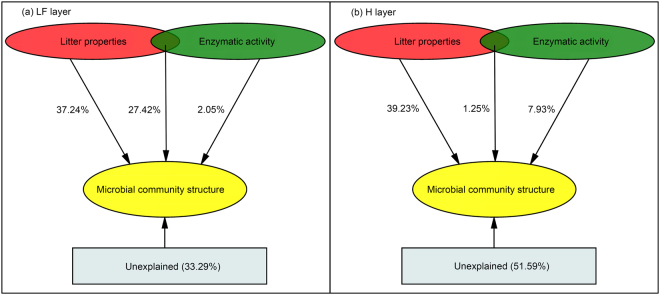
Based on a variation partitioning analysis (VPA), microbial community structure in the LF and H layers was well explained by the litter physicochemical properties and enzyme activities. In the LF and H layers, 66.71% and 48.41% of the microbial community structure, respectively, could be explained mainly by physicochemical properties (37.24% in the LF layer and 39.23% in the H layer) (Fig. 4). Only 2.03% and 7.93% of the microbial community structure in the LF layer and H layer, respectively, could be explained by enzyme activities (Fig. 4).
Figure 4.
Variation partitioning analysis (VPA) of the effects of litter properties and enzymatic activity on microbial community structure in the LF (a) and H (b) layers. Overlapping areas represent the combined effects of litter properties and enzymatic activity.
Furthermore, the BIOENV procedure identified the C:N ratio, the C:P ratio and MC as the chemical variables that were most strongly correlated with microbial community structure in the LF layer (ρ = 0.730). In contrast, pH and lignin concentrations were most strongly correlated with microbial community structure in the H layer (ρ = 0.360) (Table 4).
Table 4.
Combinations of soil variables giving the rank correlations (ρ) between chemical properties and microbial PLFA similarity matrices estimated by the BIOENV procedure (n = 45).
| LF layer | H layer | ||
|---|---|---|---|
| k | Best variable combinations (ρ) | k | Best variable combinations (ρ) |
| 1 | C:N (0.677) | 1 | Lig (0.305) |
| 2 | C:N, MC (0.711) | 2 | pH, Lig (0.360) |
| 3 | C:N, C:P, MC (0.730) | 3 | TN, pH, Lig (0.359) |
| 4 | SOC, C:N, C:P, MC (0.698) | 4 | TN, MC, pH, Lig (0.331) |
| 5 | SOC, C:N, C:P, MC, Lig (0.680) | 5 | TN, MC, pH, Cel, Lig (0.307) |
| 6 | SOC, C:N, C:P, N:P, MC, Lig (0.664) | 6 | TN, N:P, MC, pH, Cel, Lig (0.270) |
k indicates the number of soil variables. Bold type indicates the best combination overall. A total of 10 chemical variables (SOC, TN, TP, C:N, C:P, N:P, MC, pH, Lig and Cel) were included in the analysis. SOC, soil organic carbon; TN, total nitrogen; TP, total phosphorus; MC, moisture content; Cel, cellulose concentration; Lig, lignin concentration
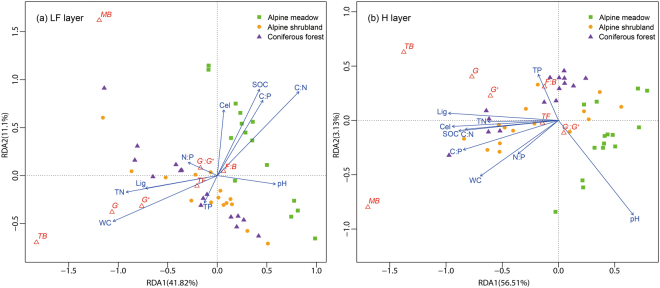
The redundancy analysis (RDA) showed that in the LF layer, the first two axes captured 52.72% of the variability of the microbial community structure, whereas RDA1 (the x-axis) and RDA2 (the y-axis) accounted for 41.62% and 11.1% of the variation, respectively (Fig. 5a). SOC, TN, C:N ratio, C:P ratio, pH, MC, cellulose concentration and lignin concentration contributed most to the separation of the samples (pH and cellulose concentration, p < 0.05; lignin concentration, p < 0.01; others, p < 0.001) (Fig. 5a and Table S1). In the H layer, the explanatory variables of litter properties and enzyme activity accounted for 59.64% of the variability in microbial community structure, with 56.51% and 3.13% of the variation explained by RDA1 and RDA2, respectively (Fig. 5b). As observed in the LF layer, SOC, TN, C:N ratio, C:P ratio, MC, cellulose concentration and lignin concentration contributed to the separation of the samples in the H layer (except TN and WC, p < 0.01; all p < 0.001) (Fig. 5b and Table S1).
Figure 5.
Redundancy analysis (RDA) of microbial indices (red hollow triangles) or litter physicochemical properties (blue lines) with the ordination scores of seven biomarker classes in the LF (a) and H (b) layers. The microbial indices are total bacteria (TB), total fungi (TF), gram-positive bacteria (G+), gram-negative bacteria (G−), fungi to bacteria ratio (F:B), ratio of gram-negative bacteria to gram-positive bacteria (G−:G+) and PLFA microbial biomass (MB). SOC, soil organic carbon; TN, total nitrogen; TP, total phosphorus; MC, moisture content; Cel, cellulose concentration; and Lig, lignin concentration.
In the LF layer, the SOC and C:N and C:P ratios were negatively correlated with the bacterial indices (TB, G+ and G−) and fungi indices, whereas MC and TN were positively correlated with these microbial indices (Fig. 5a). In the H layer, lignin concentration was positively correlated with the bacterial indices (TB, G+ and G−) and fungi indices, whereas pH was negatively correlated these microbial indices (Fig. 5b).
Discussion
Distribution of lignocellulolytic enzyme activity and microbial community structure
We found that cellulolytic (LiP and MnP) and ligninolytic (EG and BG) enzymatic activities in the LF layer were significantly higher than those in the H layer for all three vegetation types (Fig. 1). Different litter layers (fresh litter layer (L), fermentation layer (F) and humus layer (H)) can represent different stages of litter decomposition1–3. Our results clearly indicated that the activities of ligninolytic and cellulolytic enzymes decreased in the later stage of litter decomposition, similar with Snajdr et al. (2008), Papa, S., et al. (2012) and Fujii et al. (2013)1,18,19. We argue that litter nutrient content and C:N:P stoichiometry affect the structure and activity of the decomposer community20,21. In this study, the C:N, C:P, and N:P ratios; SOC; and lignin and cellulose concentrations were higher in the early stage of decomposition than in the later stage in the three vegetation types (Table 1), which may have stimulated higher enzymatic activities. The proportion of C released in the initial stage of litter decomposition is generally greater than that of N and P, and this stage tends to have higher C availability22. However, the C:N and C:P ratios decreased in the later stage of decomposition in the three vegetation types (Table 1); these decreases in the C:N and C:P ratios might have contributed to microbial carbon limitation, indicating that microbial organisms allocated fewer carbon to the production of enzymes that degrade organic matter into monosaccharides. In line with these explanations and our enzyme findings, we did observed that the biomass of microbial PLFAs (TB, TF, G+, G− and MB, Fig. 2) decreased over the process of litter decomposition. The significant differences in microbial community structure between the LF and H layers (Fig. 3) were mainly due to the differences in resource availability and the survival strategies of the microorganisms (microbial growth, metabolism, and enzyme production) between the different decomposition stages7,23. The correlation analysis revealed positive relationships between enzymatic activities and litter chemical properties (SOC and the C:N, C:P and N:P ratios) (Table 2), confirming the close association between litter substrate quality and enzymatic activity.
On a spatial scale, in general, along with the amount of total soil carbon and its readily utilizable forms, lignocellulose-degrading enzymes (and other enzymes) and microbial biomass were higher in the upper litter layers than in the humus and mineral soil layers18. The alpine treeline ecotone from coniferous forests to shrublands and alpine meadows can give rise to dramatic variations in snow depth, snowmelt time, soil temperature, soil moisture and nutrient availability, which, in combination, affect litter decomposition processes24,25. Our results showed that significant differences in lignocellulolytic enzymes and microbial characteristics between the two litter layers in the three vegetation types were similar, which could be mainly attributable to the similarity in differences in the litter characteristics (e.g., SOC and the C:N and C:P ratios) across the alpine treeline ecotone (Table 1). This vertical distribution of litter layers was reported in several forest soils ranging from the Mediterranean evergreen forests to temperate forests to boreal ecosystems1,18,19,26,27. However, in some temperate and tropical forests, Fujii et al. (2013) observed that the activities of lignin peroxidase (LiP) in the H layer were higher than in the LF layer1, which may be caused by the highly acidic H layers in that research area. The distribution of the specific basidiomycete fungi and LiP correlated with the high fungal activity in acidic and lignin-rich conditions28,29. In this study, the higher activities of LiP were in accordance with the higher lignin contents and higher fungal biomass in the LF layer (Figs 1 and 2 and Table 1).
Factors affecting microbial community structure
The BIOENV procedure indicated that the variation in microbial community structure during decomposition was mainly affected by the C:N:P stoichiometry, moisture content, pH and lignin concentration. The C:N ratio, C:P ratio and moisture content were the most important determinants of microbial community structure in the LF layer, whereas pH and lignin concentration were the major factors impacting the H layer. It is widely accepted that resource availability can contribute to microbial growth, and there is a popular notion that the C:N:P stoichiometry is strongly related to microbial community structure30–33. As discussed before, SOC and available C are more abundant in the LF layer than in the H layer. Available nutrients of low molecular weight are readily lost from the litter through dissolution and leaching in the LF layer7. Therefore, microbes prioritize immobilizing the available N and P in the LF layer34. This is consistent with our findings that TN, TP and moisture content were positively correlated with most of the bacterial and fungal indices in the LF layer (Fig. 5a). Taken together, these findings suggest that less energy (carbon)-limited and more nutrient (nitrogen or phosphorus)-conserving microbes might be responsible for the negative correlations of SOC, the C:N ratio and the C:P ratio with the bacterial and fungal indices in the LF layer (Fig. 5a). These phenomena can explain why the C:N ratio, C:P ratio and moisture content were the most important determinants of microbial community structure during the initial stage of litter decomposition. These results are consistent with our previous findings that in the early decomposition stage, the activities of β-1,4-exoglucanase and β-1,4-glucosidase appeared to be limited by the N and P contents of the substrate, whereas in the late decomposition stage, the activities of β-1,4-endoglucanase and β-1,4-glucosidase were mainly limited by the C and N contents5.
However, at later stages of decomposition, microorganisms allocate more resources to the decomposition of recalcitrant substances, such as lignin, thereby facilitating their growth and metabolism in the organic layer35,36. This mainly occurs because at an early stage of decomposition, water-soluble substances and the unshielded hemicellulose/cellulose content decrease quickly relative to the decrease in lignin due to microbial decomposition and utilization36. Furthermore, under conditions of low nutrient availability, microbial activity is more easily affected by abiotic factors7,37. A more neutral soil pH can enhance biological N fixation by increasing the availability of nutrients38. However, in the present study, we observed a relatively low pH in the H layer, particularly in coniferous forest (Table 1). Consistent with these observations, we observed that lignin concentration and pH had major influences on the microbial community structure and close associations with microbial indices in the H layer (Fig. 5b and Table 4).
Conclusions
Our results indicated that the activities of ligninolytic enzymes (LiP and MnP) and cellulolytic enzymes (EG and BG) as well as the biomass of microbial PLFAs (TB, TF, G+, G− and MB) were higher during the initial stage of litter decomposition in the three vegetation types (coniferous forest, alpine shrubland and alpine meadow). PERMANOVA demonstrated that the soil microbial community varied significantly over the course of litter decomposition in the three vegetation types. VPA demonstrated that the variation in the composition of the microbial community in the litter horizon was well explained by physicochemical properties (e.g., soil organic carbon, total nitrogen and total phosphorous) rather than by enzymatic activities. Additionally, the BIOENV procedure showed that the C:N ratio, C:P ratio and moisture content were the most important determinants of microbial community structure during the initial stage of litter decomposition, whereas pH and lignin concentration were the major factors influencing microbial community structure during the later stage. The results of RDA and BIOENV revealed that in the initial stage of litter decomposition with higher available nutrients (particularly available carbon), the microbial indices were negatively correlated with SOC, the C:N ratio and the C:P ratio, suggesting that microbes prioritize immobilizing the available N and P. In contrast, in the later stages with relatively scarce nutrients, microbes adopt strategies to utilize recalcitrant substances such as lignin and are more sensitive to non-nutrient factors, such as pH. These findings indicate that litter quality drives the differentiation of microbial communities in the litter horizon across an alpine treeline ecotone in the eastern Tibetan Plateau.
Materials and Methods
Study site and sample collection
Soil samples were collected in an alpine treeline ecotone at the Long-term Research Station of Alpine Forest Ecosystems (Zhegu Mountain region), an important riparian zone located at the eastern edge of the Tibetan Plateau in Sichuan Province, China (31°51′428′′N, 102°41′230′′E). This alpine treeline ecotone offers a natural experimental platform for studying changes in the characteristics of the microbial community during different stages of decomposition in the context of landscape-scale geologic, vegetation and climate gradients. This area contains mixed coniferous forest, broad-leaved forest, dark coniferous forest, alpine shrubland, alpine meadow from valley to hilltop, and alpine desert above 4500 m above sea level (a.s.l.); the timberline occurs at approximately 4000 m a.s.l. and is well preserved. The mountainous region is characterized by steep terrain and is highly dissected by slopes > 35°. The annual average temperature ranges from 6 to 12 °C, with average temperatures of −8 °C and 12.6 °C in January and July, respectively. The annual precipitation ranges from 600 to 1100 mm, and the annual evaporation ranges from 1000 to 1900 mm17. The dominant plant species in the coniferous forest are Minjiang fir (Abies faxoniana) and alpine rhododendron (Rhododendron taliense). The dominant shrub species in the coniferous are Rhododendron taliense, Rhododendron wiltonii, and Lonicera myrtillus. The dominant shrub species in the alpine shrubland are Salix paraplesia, Sorbus rufopilosa, Lonicera lanceolata, Rosa omeiensis, and Berberis silva-taroucana. There are clear transitions of herbaceous species from the coniferous forest to alpine shrubland and meadow. The dominant herbaceous species in the coniferous forest are Kobresia macrantha, Cystopteris moupinensis, Senecio winklerianus, and Ligularia sagitta. The dominant herbaceous species in the alpine shrubland include Epilobium angustifolium, Deyeuxia scabrescens, and Gentiana scabra. The alpine meadow is dominated by Ajuga ovalifolia, Festuca wallichanica, Polygonum paleaceum, and Pedicularis roylei. Based on United States Department of Agriculture Soil Taxonomy, the soils in the coniferous forest and shrubland are classified as Cryumbreps, and the soil type of the alpine meadow is Histosols17.
Three 50 × 200 m transects were placed along the contour (with the same mountain aspect and similar slope) in the coniferous forest (3900 to 3950 m a.s.l.), alpine shrubland (4000 to 4050 m a.s.l.), and alpine meadow (4200 to 4250 m a.s.l.). Fifteen randomly selected sample plots (plot size 2 × 2 m), each separated by more than 5 m, were placed along each transect. The LF layer is the upper 2 cm layer that contains easily recognizable plant remains that show some discoloration4,6. The 2 to 5 cm layer consists of humified material without recognizable plant structures except for stems and coarse root remains, and it is referred to as the H layer4. A total of 90 litter samples (3 transects × 2 layers × 15 plots, approximately 200 g of each sample) were collected in October 2014. The samples were immediately refrigerated at 4 °C and shipped on ice to the laboratory, where they were screened for impurities and homogenized. Samples from the LF layer were cut into approximately 0.25 cm2 pieces, whereas the samples from the H layer were sieved using a 2 mm sieve. Samples from each sample plot were divided into three subsamples: the first was frozen at −20 °C for subsequent enzymatic activity analysis; the second was frozen at −70 °C for PLFA analysis; and the third was air-dried for chemical analysis.
Soil chemical analyses
All of the samples from the LF and H layers of each transect were characterized regarding the following physicochemical properties. Moisture content (MC) was assessed using the conventional oven-drying and weighing method (105 °C for 24 h), and pH was measured using a milled litter to solution (water) ratio of 1:2.5 (w/v). The SOC content was determined using the dichromate oxidation ferrous sulfate titration method, and the TN and TP contents were determined by the Kjeldahl method and molybdenumblue colorimetry, respectively; lignin and cellulose contents were measured using the acid detergent lignin method39. The stoichiometric ratios (C:N, C:P and N:P) were calculated using SOC, TN and TP.
Enzyme extraction and assays
Ligninolytic and cellulolytic enzymatic activities that involve LiP, MnP, EG and BG were measured according to the methods of Criquet et al.40–43, with minor modifications. First, 4 to 9 g of freshly powdered litter (<0.5 mm) was extracted overnight in 15 mL of a 0.1 M CaCl2 solution containing 0.05% Tween 80 and 0.40 g of polyvinylpolypyrrolidone at 4 °C, and the suspension was centrifuged at 12000 g for 20 min at 4 °C. The supernatant was subsequently dialyzed for 48 h at 4 °C in 14-kDa molecular mass cut-off cellulose dialysis tubing against a frequently exchanged 2 mM bis-tris (bis [2-hydroxyethyl] imino-tris [hydroxymethyl] methane) buffer, pH 6.0. The extracts were boiled for 15 min to serve as controls for enzymatic activity; a reaction mixture without Mn served as a control for MnP activity. Unless otherwise indicated, all enzymatic activities were analyzed at optimal pH values (3.0, 4.5, 6.0 and 5.0 for LiP, MnP, EG and BG, respectively) and optimal temperature (Table 5). The enzyme commission (EC) number and specific substrates are provided in Table 5. One unit of enzymatic activity was defined as the amount of enzyme needed to form 1 nmol min−1 of reaction product and expressed as U g−1 dry matter {(DM = wet litter mass × (oven dry mass/wet litter mass)} (nmol min−1 g−1 DM).
Table 5.
Extracellular enzymes assayed in leaf litter, with abbreviations used in this study, enzyme commission numbers (EC), and corresponding substrates.
| Enzyme | Abbreviation | EC | Substrate | Incubation time and temperature |
|---|---|---|---|---|
| Manganese peroxidase | MnP | EC 1.11.1.13 | Phenol red | 5 min at 30 °C |
| Lignin peroxidase | LiP | EC 1.11.1.14 | Azure B | 5 min at 30 °C |
| Endo-1-4-β-glucanase | EG | EC 3.2.1.4 | Carboxymethylcellulose | 1 h at 50 °C |
| 1,4-β-glucosidase | BG | EC 3.2.1.21 | p-nitrophenyl-β-D-glucoside | 40 min at 40 °C |
Phospholipid fatty acid analysis
We investigated the soil PLFA composition to evaluate the changes in the microbial biomass with litter layer among three vegetation types and as an index of the viability of the structure of the microbial community20. Phospholipid fatty acids were extracted, fractionated and methylated as described by Bossio and Scow44, with slight modifications. A total of 1 g of fresh subsample was extracted using a mixture of chloroform, methanol and potassium phosphate buffer (1:2:0.8 v/v/v). The phospholipids in the concentrated extracts were separated on a silica gel column by sequential elution with organic solvents of increasing polarity and then saponified and methylated to form fatty acid methyl esters (FAMEs). Individual FAMEs were identified by gas chromatography/mass spectrometry (GC/MS, Model QP-2010, Shimadzu, Japan). Peak areas were converted to nanomoles per gram of dry soil (nmol g−1 DM) using internal standards (19:0 nonadecanoic methyl ester).
The following PLFAs were used as markers for specific groups: total bacteria (TB): i15:0, a15:0, 16:1ω7c, i17:0, a17:0, cy17:0 and cy19:0; total fungi (TF): 18:1ω9c and 18:2ω6c; gram-positive (G+) bacteria: i15:0, a15:0, i17:0 and a17:0; and gram-negative (G−) bacteria: 16:1ω7c, cy17:0 and cy19:044–47. The sum of all PLFAs described above together with the unspecific PLFAs (15:0, 16:0, 16:1ω5t, 17:0 and 18:0) were used to define the microbial community composition and to indicate the microbial biomass, and the ratios of fungi to bacteria (F:B) and G−:G+ were calculated48,49.
Statistical analyses
We used a Mann-Whitney U test to determine the differences in litter physicochemical properties, enzymatic activity and microbial PLFA between the two litter decomposition stages for the three vegetation types. A Pearson correlation analysis was used to evaluate the relationships between litter physicochemical properties and enzymatic activity. The Mann-Whitney U test and Pearson correlation analysis were performed using SPSS Statistics for Windows, version 20.0 (IBM Corp., Armonk, NY, USA). To visually interpret community dissimilarity, non-metric multidimensional scaling ordination (NMDS) was conducted, and PERMANOVA was performed to test whether a significant difference in bacterial community composition was present between the two litter decomposition stages in each of the three vegetation types. Both NMDS and PERMANOVA were performed based on Bray–Curtis dissimilarity (distance) of variability of all PLFA biomarkers in the samples. The redundancy analysis (RDA) (length of gradient < 3 for microbial community variables) and the BIOENV procedure (with microbial communities calculated using Bray-Curtis dissimilarity and litter chemical properties calculated using Euclidean distance) was used to identify the response of the microbial community to environmental variation. The significance of the PERMANOVA and the RDA results was tested with a Monte Carlo permutation test (permutations = 999). Microbial community structure data were analyzed in R version 3.3.2 (using the vegan package; R Development Core Team, 2011).
Electronic supplementary material
Acknowledgements
This study was financially supported by the National Natural Science Foundation of China (31570605 and 31200345).
Author Contributions
H.F.Z., Y.L. and J.Z. designed the study. W.Q.Y., Y.M.C. and L.Y. performed the experiments. H.F.Z., H.J.L. and L.F.W. analyzed the data. All of the authors were involved in discussing the data. H.F.Z. and F.Z.W. contributed to drawing the figures. H.F.Z. drafted the manuscript, and all of the authors reviewed the manuscript. H.F.Z. and L.G. approved the final version.
Competing Interests
The authors declare no competing interests.
Footnotes
Haifeng Zheng and Yamei Chen contributed equally to this work.
Electronic supplementary material
Supplementary information accompanies this paper at 10.1038/s41598-018-28150-1.
Publisher's note: Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Fujii K, Uemura M, Hayakawa C, Funakawa S, Kosaki T. Environmental control of lignin peroxidase, manganese peroxidase, and laccase activities in forest floor layers in humid Asia. Soil Biol. Biochem. 2013;57:109–115. doi: 10.1016/j.soilbio.2012.07.007. [DOI] [Google Scholar]
- 2.Hilli S, Stark S, Derome J. Carbon Quality and Stocks in Organic Horizons in Boreal Forest Soils. Ecosystems. 2008;11:270–282. doi: 10.1007/s10021-007-9121-0. [DOI] [Google Scholar]
- 3.Federer C. Subjectivity in the separation of organic horizons of the forest floor. Soil Sci. Soc. Am. J. 1982;46:1090–1093. doi: 10.2136/sssaj1982.03615995004600050041x. [DOI] [Google Scholar]
- 4.Verburg P, Van Dam D, Hefting M, Tietema A. Microbial transformations of C and N in a boreal forest floor as affected by temperature. Plant Soil. 1999;208:187–197. doi: 10.1023/A:1004462324452. [DOI] [Google Scholar]
- 5.Chen YM, et al. Litter cellulolytic enzyme activities in alpine timberline ecotone of western Sichuan. Chinese Journal of Plant Ecology. 2014;38:334–342. doi: 10.3724/SP.J.1258.2014.00114. [DOI] [Google Scholar]
- 6.Green R, Trowbridge R, Klinka K. Towards a taxonomic classification of humus forms. Forest Sci. 1993;39:a0001–z0002. [Google Scholar]
- 7.Berg, B. & Mcclaugherty, C. Plant Litter. Decomposition, Humus Formation, Carbon Sequestration. 3nd Ed, (Springer Berlin Heidelberg, 2013).
- 8.Baldrian, P. & Šnajdr, J. Soil Enzymology. 167–186 (Springer Berlin Heidelberg, 2010).
- 9.Wagner D, Kobabe S, Liebner S. Bacterial community structure and carbon turnover in permafrost-affected soils of the Lena Delta, northeastern Siberia This article is one of a selection of papers in the Special Issue on Polar and Alpine Microbiology. Can. J. Microbiol. 2009;55:73–83. doi: 10.1139/W08-121. [DOI] [PubMed] [Google Scholar]
- 10.Lopez-Mondejar R, Zuhlke D, Becher D, Riedel K, Baldrian P. Cellulose and hemicellulose decomposition by forest soil bacteria proceeds by the action of structurally variable enzymatic systems. Sci. Rep. 2016;6:25279. doi: 10.1038/srep25279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Himmel ME, et al. Microbial enzyme systems for biomass conversion: emerging paradigms. Biofuels. 2010;1:323–341. doi: 10.4155/bfs.09.25. [DOI] [Google Scholar]
- 12.Cotrufo MF, Wallenstein MD, Boot CM, Denef K, Paul E. The Microbial Efficiency‐Matrix Stabilization (MEMS) framework integrates plant litter decomposition with soil organic matter stabilization: do labile plant inputs form stable soil organic matter? Global Change Biol. 2013;19:988–995. doi: 10.1111/gcb.12113. [DOI] [PubMed] [Google Scholar]
- 13.Vrsanska M, et al. Induction of Laccase, Lignin Peroxidase and Manganese Peroxidase Activities in White-Rot Fungi Using Copper Complexes. Molecules. 2016;21:1553. doi: 10.3390/molecules21111553. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Horn SJ, Vaaje-Kolstad G, Westereng B, Eijsink V. Novel enzymes for the degradation of cellulose. Biotechnol. Biofuels. 2012;5:1. doi: 10.1186/1754-6834-5-45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.LeBauer DS. Litter degradation rate and β-glucosidase activity increase with fungal diversity. Can. J. Forest Res. 2010;40:1076–1085. doi: 10.1139/X10-054. [DOI] [Google Scholar]
- 16.Berg B. Litter decomposition and organic matter turnover in northern forest soils. Forest Ecol. Manag. 2000;133:13–22. doi: 10.1016/S0378-1127(99)00294-7. [DOI] [Google Scholar]
- 17.Liu Y, et al. Changes in foliar litter decomposition of woody plants with elevation across an alpine forest–tundra ecotone in eastern Tibet Plateau. Plant Ecol. 2016;217:495–504. doi: 10.1007/s11258-016-0594-9. [DOI] [Google Scholar]
- 18.Šnajdr J, et al. Spatial variability of enzyme activities and microbial biomass in the upper layers of Quercus petraea forest soil. Soil Biol. Biochem. 2008;40:2068–2075. doi: 10.1016/j.soilbio.2008.01.015. [DOI] [Google Scholar]
- 19.Papa S, Cembrola E, Pellegrino A, Fuggi A, Fioretto A. Microbial enzyme activities, fungal biomass and quality of the litter and upper soil layer in a beech forest of south Italy. Eur. J. Soil Sci. 2014;65:274–285. doi: 10.1111/ejss.12112. [DOI] [Google Scholar]
- 20.Schneider T, et al. Who is who in litter decomposition? Metaproteomics reveals major microbial players and their biogeochemical functions. ISME J. 2012;6:1749–1762. doi: 10.1038/ismej.2012.11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Sinsabaugh RL, Follstad Shah JJ. Ecoenzymatic stoichiometry and ecological theory. Annu. Rev. Ecol. Evol. S. 2012;43:313–343. doi: 10.1146/annurev-ecolsys-071112-124414. [DOI] [Google Scholar]
- 22.Güsewell S, Gessner MO. N: P ratios influence litter decomposition and colonization by fungi and bacteria in microcosms. Funct. Ecol. 2009;23:211–219. doi: 10.1111/j.1365-2435.2008.01478.x. [DOI] [Google Scholar]
- 23.Rosenbrock P, Buscot F, Munch J. Fungal succession and changes in the fungal degradation potential during the initial stage of litter decomposition in a black alder forest [Alnus glutinosa (L.) Gaertn.] Eur. J. Soil Biol. 1995;31:1–11. [Google Scholar]
- 24.Bharti RR, Adhikari BS, Rawat GS. Assessing vegetation changes in timberline ecotone of Nanda Devi National Park, Uttarakhand. Int. J. Appl. Earth Obs. 2012;18:472–479. doi: 10.1016/j.jag.2011.09.018. [DOI] [Google Scholar]
- 25.Sjögersten S, Wookey PA. Decomposition of mountain birch leaf litter at the forest-tundra ecotone in the Fennoscandian mountains in relation to climate and soil conditions. Plant Soil. 2004;262:215–227. doi: 10.1023/B:PLSO.0000037044.63113.fe. [DOI] [Google Scholar]
- 26.Andersson M, Kjøller A, Struwe S. Microbial enzyme activities in leaf litter, humus and mineral soil layers of European forests. Soil Biol. Biochem. 2004;36:1527–1537. doi: 10.1016/j.soilbio.2004.07.018. [DOI] [Google Scholar]
- 27.Stone M, DeForest J, Plante A. Changes in extracellular enzyme activity and microbial community structure with soil depth at the Luquillo Critical Zone Observatory. Soil Biol. Biochem. 2014;75:237–247. doi: 10.1016/j.soilbio.2014.04.017. [DOI] [Google Scholar]
- 28.Sinsabaugh RL, et al. Wood decomposition over a first-order watershed: Mass loss as a function of lignocellulase activity. Soil Biol. Biochem. 1992;24:743–749. doi: 10.1016/0038-0717(92)90248-V. [DOI] [Google Scholar]
- 29.Osono T, et al. Fungal succession and lignin decomposition on Shorea obtusa leaves in a tropical seasonal forest in northern Thailand. Fungal Divers. 2009;36:101–119. [Google Scholar]
- 30.Drenovsky RE, Vo D, Graham KJ, Scow KM. Soil water content and organic carbon availability are major determinants of soil microbial community composition. Microb. Ecol. 2004;48:424–430. doi: 10.1007/s00248-003-1063-2. [DOI] [PubMed] [Google Scholar]
- 31.Bausenwein U, et al. Exploring soil microbial communities and soil organic matter: variability and interactions in arable soils under minimum tillage practice. Appl. Soil Ecol. 2008;40:67–77. doi: 10.1016/j.apsoil.2008.03.006. [DOI] [Google Scholar]
- 32.Brockett BFT, Prescott CE, Grayston SJ. Soil moisture is the major factor influencing microbial community structure and enzyme activities across seven biogeoclimatic zones in western Canada. Soil Biol. Biochem. 2012;44:9–20. doi: 10.1016/j.soilbio.2011.09.003. [DOI] [Google Scholar]
- 33.Zhang XF, et al. Soil moisture effect on bacterial and fungal community in Beilu River (Tibetan Plateau) permafrost soils with different vegetation types. Journal of applied microbiology. 2013;114:1054–1065. doi: 10.1111/jam.12106. [DOI] [PubMed] [Google Scholar]
- 34.Manzoni S, Trofymow JA, Jackson RB, Porporato A. Stoichiometric controls on carbon, nitrogen, and phosphorus dynamics in decomposing litter. Ecol. Monogr. 2010;80:89–106. doi: 10.1890/09-0179.1. [DOI] [Google Scholar]
- 35.Laskowski, R. & Berg, B. Litter decomposition: guide to carbon and nutrient turnover. 421 (Amsterdam, 2006).
- 36.Couteaux M-M, Bottner P, Berg B. Litter decomposition, climate and liter quality. Trends Ecol. Evol. 1995;10:63–66. doi: 10.1016/S0169-5347(00)88978-8. [DOI] [PubMed] [Google Scholar]
- 37.Vries FT, et al. Abiotic drivers and plant traits explain landscape‐scale patterns in soil microbial communities. Ecol. Lett. 2012;15:1230–1239. doi: 10.1111/j.1461-0248.2012.01844.x. [DOI] [PubMed] [Google Scholar]
- 38.Delgado-Baquerizo M, et al. Decoupling of soil nutrient cycles as a function of aridity in global drylands. Nature. 2013;502:672–676. doi: 10.1038/nature12670. [DOI] [PubMed] [Google Scholar]
- 39.Vanderbilt K, White C, Hopkins O, Craig J. Aboveground decomposition in arid environments: results of a long-term study in central New Mexico. J. Arid Environ. 2008;72:696–709. doi: 10.1016/j.jaridenv.2007.10.010. [DOI] [Google Scholar]
- 40.Criquet S, Tagger S, Vogt G, Iacazio G, Le Petit J. Laccase activity of forest litter. Soil Biol. Biochem. 1999;31:1239–1244. doi: 10.1016/S0038-0717(99)00038-3. [DOI] [Google Scholar]
- 41.Criquet S. Measurement and characterization of cellulase activity in sclerophyllous forest litter. J. Microbiol. Meth. 2002;50:165–173. doi: 10.1016/S0167-7012(02)00028-3. [DOI] [PubMed] [Google Scholar]
- 42.Valášková V, et al. Production of lignocellulose-degrading enzymes and degradation of leaf litter by saprotrophic basidiomycetes isolated from a Quercus petraea forest. Soil Biol. Biochem. 2007;39:2651–2660. doi: 10.1016/j.soilbio.2007.05.023. [DOI] [Google Scholar]
- 43.Arora DS, Chander M, Gill PK. Involvement of lignin peroxidase, manganese peroxidase and laccase in degradation and selective ligninolysis of wheat straw. Int. Biodeter. Biodegr. 2002;50:115–120. doi: 10.1016/S0964-8305(02)00064-1. [DOI] [Google Scholar]
- 44.Bossio DA, Scow KM. Impacts of carbon and flooding on soil microbial communities: phospholipid fatty acid profiles and substrate utilization patterns. Microb. Ecol. 1998;35:265–278. doi: 10.1007/s002489900082. [DOI] [PubMed] [Google Scholar]
- 45.Kourtev PS, Ehrenfeld JG, Häggblom M. Experimental analysis of the effect of exotic and native plant species on the structure and function of soil microbial communities. Soil Biol. Biochem. 2003;35:895–905. doi: 10.1016/S0038-0717(03)00120-2. [DOI] [Google Scholar]
- 46.Frostegård Å, Tunlid A, Bååth E. Use and misuse of PLFA measurements in soils. Soil Biol. Biochem. 2011;43:1621–1625. doi: 10.1016/j.soilbio.2010.11.021. [DOI] [Google Scholar]
- 47.Kaiser C, et al. Belowground carbon allocation by trees drives seasonal patterns of extracellular enzyme activities by altering microbial community composition in a beech forest soil. New Phytol. 2010;187:843–858. doi: 10.1111/j.1469-8137.2010.03321.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Zelles L. Fatty acid patterns of phospholipids and lipopolysaccharides in the characterisation of microbial communities in soil: a review. Biol. Fertil. Soils. 1999;29:111–129. doi: 10.1007/s003740050533. [DOI] [Google Scholar]
- 49.Mooshammer M, et al. Decoupling of microbial carbon, nitrogen, and phosphorus cycling in response to extreme temperature events. Sci. Adv. 2017;3:e1602781. doi: 10.1126/sciadv.1602781. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.