Abstract
Postmenopausal osteoporosis (PMOP) is a common systemic skeletal disease characterized by reduced bone mass and microarchitecture deterioration. Although differentially expressed SOX5 has been found in bone marrow from ovariectomized mice, its role in osteogenic differentiation in human mesenchymal stem cells (hMSCs) from bone marrow in PMOP remains unknown. In this study, we investigated the biological function of SOX5 and explore its molecular mechanism in hMSCs from patients with PMOP. Our findings showed that the mRNA and protein expression levels of SOX5 were upregulated in hMSCs isolated from bone marrow samples of PMOP patients. We also found that SOX5 overexpression decreased the alkaline phosphatase (ALP) activity and the gene expression of osteoblast markers including Collagen I, Runx2 and Osterix, which were increased by SOX5 knockdown using RNA interference. Furthermore, TNF-α notably upregulated the SOX5 mRNA expression level, and SOX5 knockdown reversed the effect of TNF-α on osteogenic differentiation of hMSCs. In addition, SOX5 overexpression increased Kruppel-like factor 4 (KLF4) gene expression, which was decreased by SOX5 silencing. KLF4 knockdown abrogated the suppressive effect of SOX5 overexpression on osteogenic differentiation of hMSCs. Taken together, our results indicated that TNF-α-induced SOX5 upregulation inhibited osteogenic differentiation of hMSCs through KLF4 signal pathway, suggesting that SOX5 might be a novel therapeutic target for PMOP treatment.
Keywords: human mesenchymal stem cells, KLF4, osteogenic differentiation, postmenopausal osteoporosis, SOX5
INTRODUCTION
Postmenopausal osteoporosis (PMOP), which occurs in postmenopausal women, is a common systemic skeletal disease characterized by reduced bone mass and deterioration of microarchitecture (Black and Rosen, 2016). Its most prevalent complication is a fragility fracture, with the most severe often leading to permanent disability, recurrent hospitalizations, and significant mortality (Lotters et al., 2016; Silva and Branco, 2012). Although medications for PMOP such as bisphosphonates, calcitonin and selective estrogen receptor modulators are used to prevent bone loss and increase bone mineral density, these medicines are inadequate and serious side effects (Xu et al., 2017). The optimal option still need to be investigated. In addition, it has been known that estrogen deficiency induces an imbalance in bone formation and bone absorption, and it plays a central role in the etiology of PMOP (Sapir-Koren and Livshits, 2017). Recent reports have indicated that estrogen deficiency upregulated the production of inflammation factors such as IL-1, IL-6 and TNF-α, which also affected bone resorption and bone formation (Kameda et al., 2015; Raehtz et al., 2017). However, the detailed mechanism of postmenopausal osteoporosis is still unknown.
Human mesenchymal stem cells (hMSCs) are non-hematopoietic multipotent cells that self-renew and have diverse differentiation potential (Abdallah and Kassem, 2008). These cells can be isolated from adult tissues (e.g., bone marrow, adipose tissue, and umbilical cord and placenta) (Ding et al., 2011). Under certain conditions, hMSCs can differentiate into a plethora of different mature cell lineages including osteogenic, chondrogenic, adipogenic, myogenic and fibroblastic (Udalamaththa et al., 2016). Thus, hMSCs have served as ideal seed cells for regenerating various tissues in treating incurable diseases. However, abnormal hMSCs are closely related to osteoporosis (Wang et al., 2016). The osteogenic differentiation capability of hMSCs from postmenopausal women with osteoporosis is lower than that those from healthy volunteers (Rodriguez et al., 2000). Therefore, improving osteogenic differentiation of hMSCs may be an important method for treating PMOP.
Sex determining region Y-box protein 5 (SOX5) (Dy et al., 2008; Wegner, 1999) is a member of the SoxD group of the SOX family, which comprises ten groups (A-J) and encods various transcription factors controlling cell fate and differentiation in many lineages (Feng et al., 2016) including the spermatids, neurons, chondrocytes, and B cells. SOX5 also participates in the progression of various cancers (Wang et al., 2015) and Type 2 diabetes (Axelsson et al., 2017). Recent studies indicated that SOX5 was closely related to elderly onset rheumatoid arthritis (Wang and Zhao, 2017) and chondrogenesis (Wegner, 2010). Moreover, differentially expressed SOX5 has been found in bone marrow cells from ovariectomized (OVX) mice (Pineda et al., 2014). Whether SOX5 functions during the osteogenic differentiation of hMSCs in PMOP is still unknown. Therefore, the molecular function of SOX5 and its mechanism in PMOP must be investigated.
In this study, we detected SOX5 gene expression in hMSCs of bone marrow samples from patients with PMOP and from healthy premenopausal women. We found that the mRNA and protein expression levels of SOX5 were notably upregulated in PMOP group compared with the healthy control. Moreover, SOX5 overexpression inhibited osteogenic differentiation of hMSCs through the Kruppel-like factor 4 (KLF4) signal pathway. Taken together, our fingdings indicate that SOX5 is a promising molecular target for PMOP treatment.
MATERIALS AND METHODS
Cell culture
As previously reported (Alm et al., 2012; Mariner et al., 2012), hMSCs were isolated from bone marrow samples, and collected from patients with PMOP( mean age of 46.5 years) and from healthy premenopausal women (healthy control, mean aged 45.8 years) in our hospital between November 2016 and March 2017. Informed consent was obtained from each participant as a donor of bone marrow. The diagnosis of osteoporosis complied with World Health Organization parameters. Subjects with a medical history of osteoporosis treatment, acute inflammation of the gastrointestinal tract, and illnesses that could affect bone metabolism were excluded. Isolated hMSCs were cultured in MSC medium containing α-modified essential medium supplemented with 10% fetal bovine serum, 2 mM of L-glutamine, 100 U/ml penicillin, and 100 mg/ml of streptomycin. Cells were maintained in humidified air with 5% CO2 at 37°C.
Cell transfection
The recombinant plasmid pcDNA3.1/SOX5 and negative control plasmid (pcontrol) were designed by Genepharma (China). siRNAs (SOX5 and KLF4), and their negative control (siNC) were purchased from RiboBio (China). The three passages hMSCs were transfected with the previously described plasmid or siRNAs using Lipofectamine 3000 (Invitrogen) according to the manufacturer’s protocol.
Osteoblast differentiation
The hMSCs transfected with the previously described plasmid or siRNAs were cultured for 14 days in osteogenic medium (OM) containing TNF-α. The OM contained the previously described MSC medium, 10 nM dexamethasone, 20 mM β-glycerophosphate, and 50 μg/ml L-ascorbic acid. Cells maintained in OM without TNF-α served as a control group.
Alkaline phosphatase (ALP) activity assay
ALP is an early marker of osteoblast differentiation. ALP activity was examined by QuantiChrom ALP Assay Kit (BioAssay Systems, USA) according to the manufacturer’s protocol. Briefly, the transfected hMSCs were cultured with OM for 14 days. Then, the OM medium was removed, and cells were lysed using a cell lysis buffer followed by centrifugation at 12,000 g force for 10 min. Besides, the protein concentration for each sample was determined with the Bradford method. Next, the resulting supernatants were incubated with p-nitrophenol phosphate at 37°C for 30 min at room temperature. Absorbance at 405 nm for each well was measured with a microplate reader.
Quantitative real-time polymerase chain reaction (qRT-PCR)
Total RNA from cultured cells was extracted using Trizol reagent (Invitrogen, USA) according to the manufacturer’s protocol. Then, 1 μg of RNA was used to synthesize cDNA using the Superscript II reverse transcriptase (Invitrogen-Life Technologies, UK). Next, RT-PCR was performed using the SYBR Premix Ex Taq II kit (TaKaRa) in the Bio-Rad IQ5 RT-PCR system according to the manufacturer’s instructions. Relative fold changes in mRNA were analyzed by using the 2−ΔΔCT method. β-actin served as the internal control.
Western blotting assay
Total protein was extracted from cells by radioimmunoprecipitation assay buffer containing protease inhibitors. The protein concentration was detected using a BCA protein assay (BioRad, USA). Then, equal amounts of protein were loaded and separated on sodium dodecyl sulfate-polyacrylamide gel electrophoresis, and then transferred onto nitrocellulose membranes. After blocking with 5% non-fat milk, the membranes were incubated overnight at 4°C with the following specific primary antibodies: anti-SOX5, anti-Collagen I, anti-Runx2, anti-Osterix, and anti-KLF4 (Sigma-Aldrich, USA), and anti-β-actin antibody (Sigma, USA). After incubation with a peroxidase-conjugated secondary antibodies (Sigma) for 2 h at room temperature, the target proteins were visualized using enhanced chemiluminescence reagents (Pierce, USA) on Image Reader LAS-3000 Fujifilm. The density of the bands on the membrane were quantified with Image J software. β-actin was used as an internal control.
Statistical analysis
Each experiment was repeated three times. SPSS 16.0 software was used for statistical analysis. All data in figures were provided as means ± SD. Statistical comparisons were made between two groups with a t-test. P <0.05 were considered significant.
RESULTS
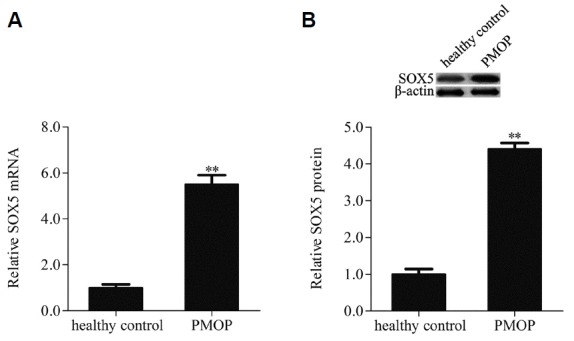
SOX5 was up-regulated in patients with postmenopausal osteoporosis
In order to investigate the biological function of SOX5 in postmenopausal osteoporosis, SOX5 gene expression was examined by qRT-PCR and Western blotting in hMSCs isolated from bone marrow samples of patients with postmenopausal osteoporosis (PMOP) and healthy premenopausal women (healthy control). Results indicated that the mRNA expression of SOX5 was notably upregulated when compared with healthy control (Fig. 1A). Additionally, SOX5 protein expression level was higher in the PMOP group than in control group (Fig.1B). These findings indicated that SOX5 was involved in the progression of postmenopausal osteoporosis.
Fig. 1. SOX5 was up-regulated in hMSCs isolated from bone marrow samples of patients with postmenopausal osteoporosis (PMOP) and healthy premenopausal women (healthy control), the mRNA and protein expression levels of SOX5 were examined by qRT-PCR (A) and Western blotting (B). Data are expressed as the mean ± SD, n=3. **P < 0.01vs. healthy control.
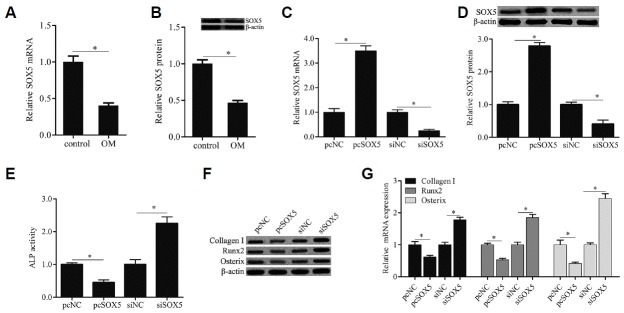
SOX5 overexpression inhibited osteogenic differentiation of hMSCs
To explore the effect of SOX5 on osteogenic differentiation of hMSCs, SOX5 gene expression was detected during osteogenic differentiation of hMSCs isolated from healthy premenopausal women. As shown in Figs. 2A and 2B, after cells culturing in OM for 14 day (OM), the mRNA and protein expresion levels of SOX5 were remarkably decreased when compared with cells at 0 day (control). Moreover, hMSCs were transfected with the recombinant plasmid pcDNA3.1/SOX5 or SOX5 siRNA, and the efficiency of transfection was evaluated by qRT-PCR and Western blotting (Figs. 2C and 2D). In addition, compared with siNC control, SOX5 overexpression remarkably diminished ALP activity (Fig. 2E) and the gene expression of osteoblast markers including Collagen I, Runx2 and Osterix (Figs. 2F and 2G), which were notably increased by SOX5 knockdown. Taken together, our findings indicated that SOX5 overexpression inhibited osteogenic differentiation of hMSCs.
Fig. 2. SOX5 overexpression inhibited osteogenic differentiation of hMSCs.
SOX5 mRNA and protein expression were examined by qRTPCR (A) and western blotting (B) in cells cultured with OM for 0 days (control) and 14 days (OM). Cells were transfected with recombinant plasmid pcDNA3.1/SOX5 (pcSOX5) and its negative control plasmid (pcNC), or SOX5 siRNA (siSOX5) and its negative control (siNC) for 15 h, after which the medium was replaced with OM for 14 days. The efficiency of transfection was evaluated by qRT-PCR (C) and Western blotting (D). ALP activity was detected by a commercial Alkaline Phosphatase Detection Kit (E). The genes expression of osteogenic marker including Collagen I, Runx2 and Osterix were examined by Western blotting (F) and qRT-PCR (G). Data are expressed as the mean ± SD, n = 3. *P < 0.05.
TNF-α induced SOX5 gene expression during osteogenic differentiation of hMSCs
To investigate whether TNF-α regulates SOX5 gene expression during osteogenic differentiation of hMSCs, SOX5 gene expression was examined by qRT-PCR and Western blotting. Our findings showed that TNF-α notably upregulated the SOX5 mRNA expression level in hMSCs with OM treatment (Fig. 3A). In addition, compared with hMSCs with OM treatment alone, the protein expression level of SOX5 was remarkably increased in cells with OM and TNF-α treatment (Fig. 3B). Moreover, in hMSCs with OM treatment, SOX5 knockdown reversed the effect of TNF-α on ALP activity (Fig. 3C) and the gene expression of osteoblast markers (Figs. 3D and 3E). These data suggested that TNF-α induced SOX5 gene expression during osteogenic differentiation of hMSCs.
Fig. 3. TNF-α induced SOX5 gene expression during osteogenic differentiation of hMSCs.
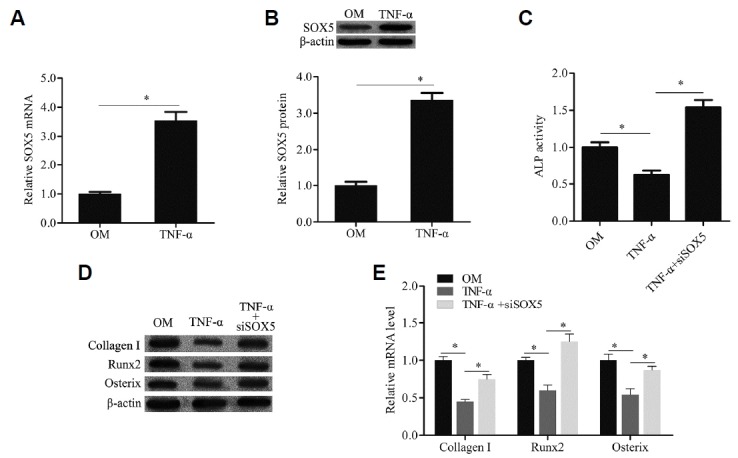
The SOX5 mRNA and protein expression were examined by qRT-PCR (A) and western blotting (B). ALP activity was detected by a commercial Alkaline Phosphatase Detection Kit (C). The genes expression of osteogenic marker including Collagen I, Runx2 and Osterix were examined by Western blotting (D) and qRTPCR (E). TNF-α: cells were cultured with OM containing TNF-α; TNF-α+siSOX5: cells were transfected with SOX5 siRNA and then cultured with OM containing TNF-α. Data are expressed as the mean ± SD, n = 3. *P < 0.05.
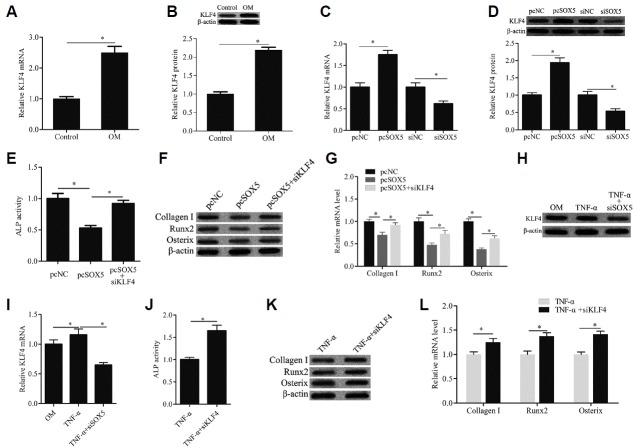
SOX5 mediated osteogenic differentiation of hMSCs through KLF4 signal pathway
To further investigate the molecular mechanism of SOX5 mediating osteogenic differentiation of hMSCs, the gene expression of KLF4 was detected. The data of qRT-PCR and Western blotting showed that the mRNA and protein expression levels of KLF4 were notably upregulated during osteogenic differentiation of hMSCs (Figs. 4A and 4B). SOX5 overexpression remarkably increased KLF4 gene expression, which was significantly decreased by SOX5 silencing (Figs. 4C and 4D). Moreover, KLF4 knockdown abrogated the suppressive effect of SOX5 overexpression on ALP activity (Fig. 4E) and the gene expression of Collagen I, Runx2 and Osterix (Figs. 4F and 4G). In addition, we found that SOX5 knockdown also significantly decreased the KLF4 gene expression compared with that of the TNF-α group (Figs. 4H and 4I). SOX5 knockdown also abrogated the suppressive effect of TNF-α on ALP activity (Fig. 4J) and the gene expression of Collagen I, Runx2 and Osterix (Figs. 4K and 4L). These findings indicated that SOX5 mediated osteogenic differentiation of hMSCs through KLF4 signal pathway.
Fig. 4. SOX5 mediated osteogenic differentiation of hMSCs through KLF4 signal pathway.
The KLF4 mRNA and protein expression were examined by qRT-PCR (A, C, and I) and western blotting (B, D, and H). ALP activity was detected by a commercial Alkaline Phosphatase Detection Kit (E, J). The genes expression of osteogenic marker including Collagen I, Runx2 and Osterix were examined by Western blotting (F, K) and qRT-PCR (G, L). pcSOX5+siKLF4: cells were transfected with recombinant plasmid pcDNA3.1/SOX5 (pcSOX5) and KLF4 siRNA, and then cultured with OM. TNF-α+siKLF4: cells were transfected with KLF4 siRNA and then cultured with OM containing TNF-α. Data are expressed as the mean ± SD, n = 3. *P < 0.05.
DISCUSSION
In the present study, we found that the mRNA and protein expression levels of SOX5 were notably upregulated in hMSCs isolated from bone marrow samples of patients with PMOP. Moreover, SOX5 overexpression remarkably diminished the ALP activity and gene expression of osteoblast markers including Collagen I, Runx2 and Osterix, which were notably increased by SOX5 knockdown. Furthermore, during osteogenic differentiation of hMSCs, TNF-α induced SOX5 gene expression. In addition, the mRNA and protein expression levels of KLF4 were notably upregulated during osteogenic differentiation of hMSCs. SOX5 overexpression remarkably increased the KLF4 gene expression, which was significantly decreased by SOX5 silencing. KLF4 knockdown abrogated the suppressive effect of SOX5 overexpression on ALP activity and gene expression of Collagen I, Runx2 and Osterix. Taken together, our results indicated that TNFα-induced SOX5 upregulation inhibited osteogenic differentiation of hMSCs through the KLF4 signaling pathway. These findings suggested that SOX5 is involved in the occurrence and development of PMOP.
Previous studies showed that hMSCs played an important role in bone metabolism, and their differentiation also played a central role in the development of PMOP (Lv et al., 2015; Yao et al., 2013; Zhao et al., 2011). Many differentially expressed genes have been reported in bone marrow hMSCs in OVX animal model or patient with PMOP (Pineda et al., 2014; Song et al., 2017). However, the detailed molecular mechanism of these genes regulating osteogenic differentiation of hMSCs in PMOP is still not well understood. In our study, the upregulation of SOX5 gene expression was found in hMSCs isolated from bone marrow samples of patients with PMOP, which was in line with a previous report in an OVX rat model (Pineda et al., 2014). We further found that SOX5 overexpression inhibited osteogenic differentiation of hMSCs. These findings provide an evidence for the potential role of SOX5 in regulating osteogenic differentiation of hMSCs in PMOP.
Inflammatory cytokines, especially TNF-α, affect the osteogenic differentiation of hMSCs and impair bone formation in osteoporosis (Pacifici, 1996; Sang et al., 2016). It has been reported that TNF-α levels are increased in patients with PMOP and OVX mice (Yang et al., 2013). Feng X et al found that SOX5 level was higher in synovium and synovial fluid from rheumatoid arthritis compared to osteoarthritis patients (Feng et al., 2016). Thus, we predicted that TNF-α may regulate the SOX5 gene expression and further affect the osteoblast differentiation of hMSCs in PMOP. Results comfirmed that TNF-α notably upregulates the SOX5 mRNA and protein expression levels in hMSCs with OM treatment. SOX5 knockdown reverses the effect of TNF-α on ALP activity and the gene expression of osteoblast markers. Our results might provide a novel mechanism in which TNF-α inhibits osteoblast differentiation of hMSCs by upregulating SOX5 expression in estrogen deficiency-induced osteoporosis.
KLF4, also known as gut-enriched KLF or epithelial zinc finger protein, is expressed in various tissues including the epithelia of the intestine, thymus, and skin. KLF4 plays an important role in cell differentiation and homeostasis in diverse diseases. Tiwari A et al indicated that KLF4 promoted corneal epithelial cell fate by inhibiting epithelial-mesenchymal transitio, and played an essential role in corneal epithelial homeostasis (Tiwari et al., 2017). Moreover, KLF4 functions as a tumor suppressor or oncogene to mediate cell growth and metastasis in different tumor type (Li et al., 2015; Nosho et al., 2007; Ohnishi et al., 2003; Tai et al., 2011). In addition, Kim et al. (2014) reported that KLF4 mediated bone homeostasis by inhibiting osteoclast formation and osteoblast differentiation. SOX5 promotes EMT and cell invasion of liver cancer via regulation of Twist1 (Wang et al., 2015), which induces endothelial differentiation of tumour cells through the Jagged1-KLF4 axis (Chen et al., 2014). However, in osteogenic differentiation of hMSCs, whether SOX5 mediates the KLF4 signaling pathway is still unknown. Therefore, in our study, we examined the KLF4 gene expression using qRT-PCR and Western blotting. Results showed that KLF4 gene expression was notably upregulated during osteogenic differentiation of hMSCs. SOX5 overexpression remarkably increased KLF4 gene expression. We also found that KLF4 knockdown abrogates the suppressive effect of SOX5 overexpression on osteogenic differentiation of hMSCs. These findings indicated that SOX5 mediated osteogenic differentiation of hMSCs through the KLF4 signaling pathway.
Moreover, whether SOX5 regulates KLF4 expression through direct binding the promoter region of KLF4 or indirect connection with other transcription factors is still not clear and would be required for further research. Interestingly, in PMOP, estrogen deficiency upregulates production of TNF-α, we found that TNF-α induced SOX5 gene expression during osteogenic differentiation of hMSCs in vitro. Whether estrogen deficiency could directly affect the SOX5 gene expression? This question is still further investigated. In addition, to further confirm the biological function of SOX5 in estrogen deficiency-induced osteoporosis, the effect of SOX5 on osteogenic differentiation and bone formation in the OVX rat model would be also required for research.
In conclusion, this study indicated for the first time that SOX5 gene expression was upregulated in hMSCs isolated from bone marrow samples from patients with PMOP. SOX5 overexpression inhibited osteogenic differentiation of hMSCs through the KLF4 signaling pathway. Furthermore, TNF-α induced SOX5 gene expression during osteogenic differentiation of hMSCs. These results suggest that SOX5 may be a novel potential diagnostic and therapeutic target for PMOP treatment.
REFERENCES
- Abdallah B.M., Kassem M. Human mesenchymal stem cells: from basic biology to clinical applications. Gene Ther. 2008;15:109–116. doi: 10.1038/sj.gt.3303067. [DOI] [PubMed] [Google Scholar]
- Alm J.J., Heino T.J., Hentunen T.A., Vaananen H.K., Aro H.T. Transient 100 nM dexamethasone treatment reduces inter-and intraindividual variations in osteoblastic differentiation of bone marrow-derived human mesenchymal stem cells. Tissue Eng Part C Methods. 2012;18:658–666. doi: 10.1089/ten.TEC.2011.0675. [DOI] [PubMed] [Google Scholar]
- Axelsson A.S., Mahdi T., Nenonen H.A., Singh T., Hanzelmann S., Wendt A., Bagge A., Reinbothe T.M., Millstein J., Yang X., et al. Sox5 regulates beta-cell phenotype and is reduced in type 2 diabetes. Nat Commun. 2017;8:15652. doi: 10.1038/ncomms15652. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Black D.M., Rosen C.J. Clinical Practice. Postmenopausal Osteoporosis. N Engl J Med. 2016;374:254–262. doi: 10.1056/NEJMcp1513724. [DOI] [PubMed] [Google Scholar]
- Chen H.F., Huang C.H., Liu C.J., Hung J.J., Hsu C.C., Teng S.C., Wu K.J. Twist1 induces endothelial differentiation of tumour cells through the Jagged1-KLF4 axis. Nat Commun. 2014;5:4697. doi: 10.1038/ncomms5697. [DOI] [PubMed] [Google Scholar]
- Ding D.C., Shyu W.C., Lin S.Z. Mesenchymal stem cells. Cell Transplant. 2011;20:5–14. doi: 10.3727/096368910X. [DOI] [PubMed] [Google Scholar]
- Dy P., Han Y., Lefebvre V. Generation of mice harboring a Sox5 conditional null allele. Genesis. 2008;46:294–299. doi: 10.1002/dvg.20392. [DOI] [PubMed] [Google Scholar]
- Feng X., Shi Y., Xu L., Peng Q., Wang F., Wang X., Sun W., Lu Y., Tsao B.P., Zhang M., et al. Modulation of IL-6 induced RANKL expression in arthritic synovium by a transcription factor SOX5. Sci Rep. 2016;6:32001. doi: 10.1038/srep32001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kameda Y., Takahata M., Mikuni S., Shimizu T., Hamano H., Angata T., Hatakeyama S., Kinjo M., Iwasaki N. Siglec-15 is a potential therapeutic target for postmenopausal osteoporosis. Bone. 2015;71:217–226. doi: 10.1016/j.bone.2014.10.027. [DOI] [PubMed] [Google Scholar]
- Kim J.H., Kim K., Youn B.U., Lee J., Kim I., Shin H.I., Akiyama H., Choi Y., Kim N. Kruppel-like factor 4 attenuates osteoblast formation, function, and cross talk with osteoclasts. J Cell Biol. 2014;204:1063–1074. doi: 10.1083/jcb.201308102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li W., Liu M., Su Y., Zhou X., Liu Y., Zhang X. The Janus-faced roles of Kruppel-like factor 4 in oral squamous cell carcinoma cells. Oncotarget. 2015;6:44480–44494. doi: 10.18632/oncotarget.6256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lotters F.J., van den Bergh J.P., de Vries F., Rutten-van Molken M.P. Current and Future Incidence and Costs of Osteoporosis-Related Fractures in The Netherlands: Combining Claims Data with BMD Measurements. Calcif Tissue Int. 2016;98:235–243. doi: 10.1007/s00223-015-0089-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lv H., Sun Y., Zhang Y. MiR-133 is involved in estrogen deficiency-induced osteoporosis through modulating osteogenic differentiation of mesenchymal stem cells. Med Sci Monit. 2015;21:1527–1534. doi: 10.12659/MSM.894323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mariner P.D., Johannesen E., Anseth K.S. Manipulation of miRNA activity accelerates osteogenic differentiation of hMSCs in engineered 3D scaffolds. J Tissue Eng Regen Med. 2012;6:314–324. doi: 10.1002/term.435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nosho K., Yamamoto H., Takahashi T., Mikami M., Taniguchi H., Miyamoto N., Adachi Y., Arimura Y., Itoh F., Imai K., et al. Genetic and epigenetic profiling in early colorectal tumors and prediction of invasive potential in pT1 (early invasive) colorectal cancers. Carcinogenesis. 2007;28:1364–1370. doi: 10.1093/carcin/bgl246. [DOI] [PubMed] [Google Scholar]
- Ohnishi S., Ohnami S., Laub F., Aoki K., Suzuki K., Kanai Y., Haga K., Asaka M., Ramirez F., Yoshida T. Downregulation and growth inhibitory effect of epithelial-type Kruppel-like transcription factor KLF4, but not KLF5, in bladder cancer. Biochem Biophys Res Commun. 2003;308:251–256. doi: 10.1016/s0006-291x(03)01356-1. [DOI] [PubMed] [Google Scholar]
- Pacifici R. Estrogen, cytokines, and pathogenesis of postmenopausal osteoporosis. J Bone Miner Res. 1996;11:1043–1051. doi: 10.1002/jbmr.5650110802. [DOI] [PubMed] [Google Scholar]
- Pineda B., Serna E., Laguna-Fernandez A., Noguera I., Panach L., Hermenegildo C., Tarin J.J., Cano A., Garcia-Perez M.A. Gene expression profile induced by ovariectomy in bone marrow of mice: a functional approach to identify new candidate genes associated to osteoporosis risk in women. Bone. 2014;65:33–41. doi: 10.1016/j.bone.2014.05.001. [DOI] [PubMed] [Google Scholar]
- Raehtz S., Bierhalter H., Schoenherr D., Parameswaran N., McCabe L.R. Estrogen deficiency exacerbates type 1 diabetes-induced bone TNF-alpha expression and osteoporosis in female mice. Endocrinology. 2017;158:2086–2101. doi: 10.1210/en.2016-1821. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rodriguez J.P., Montecinos L., Rios S., Reyes P., Martinez J. Mesenchymal stem cells from osteoporotic patients produce a type I collagen-deficient extracellular matrix favoring adipogenic differentiation. J Cell Biochem. 2000;79:557–565. doi: 10.1002/1097-4644(20001215)79:4<557::aid-jcb40>3.0.co;2-h. [DOI] [PubMed] [Google Scholar]
- Sang C., Zhang Y., Chen F., Huang P., Qi J., Wang P., Zhou Q., Kang H., Cao X., Guo L. Tumor necrosis factor alpha suppresses osteogenic differentiation of MSCs by inhibiting semaphorin 3B via Wnt/beta-catenin signaling in estrogen-deficiency induced osteoporosis. Bone. 2016;84:78–87. doi: 10.1016/j.bone.2015.12.012. [DOI] [PubMed] [Google Scholar]
- Sapir-Koren R., Livshits G. Postmenopausal osteoporosis in rheumatoid arthritis: The estrogen deficiency-immune mechanisms link. Bone. 2017;103:102–115. doi: 10.1016/j.bone.2017.06.020. [DOI] [PubMed] [Google Scholar]
- Silva I., Branco J.C. Denosumab: recent update in postmenopausal osteoporosis. Acta Reumatol Port. 2012;37:302–313. [PubMed] [Google Scholar]
- Song I., Choi Y.J., Jin Y., Kim J.W., Koh J.T., Ji H.M., Jeong S.Y., Won Y.Y., Kim W., Chung Y.S. STRA6 as a possible candidate gene for pathogenesis of osteoporosis from RNAseq analysis of human mesenchymal stem cells. Mol Med Rep. 2017;16:4075–4081. doi: 10.3892/mmr.2017.7072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tai S.K., Yang M.H., Chang S.Y., Chang Y.C., Li W.Y., Tsai T.L., Wang Y.F., Chu P.Y., Hsieh S.L. Persistent Kruppel-like factor 4 expression predicts progression and poor prognosis of head and neck squamous cell carcinoma. Cancer Sci. 2011;102:895–902. doi: 10.1111/j.1349-7006.2011.01859.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tiwari A., Loughner C.L., Swamynathan S., Swamynathan S.K. KLF4 plays an essential role in corneal epithelial homeostasis by promoting epithelial cell fate and suppressing epithelial-mesenchymal transition. Invest Ophthalmol Vis Sci. 2017;58:2785–2795. doi: 10.1167/iovs.17-21826. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Udalamaththa V.L., Jayasinghe C.D., Udagama P.V. Potential role of herbal remedies in stem cell therapy: proliferation and differentiation of human mesenchymal stromal cells. Stem Cell Res Ther. 2016;7:110. doi: 10.1186/s13287-016-0366-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang J., Zhao Q. Expression of CCR3, SOX5 and LC3 in patients with elderly onset rheumatoid arthritis and the clinical significance. Exp Ther Med. 2017;14:3573–3576. doi: 10.3892/etm.2017.4961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang D., Han S., Wang X., Peng R., Li X. SOX5 promotes epithelial-mesenchymal transition and cell invasion via regulation of Twist1 in hepatocellular carcinoma. Med Oncol. 2015;32:461. doi: 10.1007/s12032-014-0461-2. [DOI] [PubMed] [Google Scholar]
- Wang C., Meng H., Wang X., Zhao C., Peng J., Wang Y. Differentiation of bone marrow mesenchymal stem cells in osteoblasts and adipocytes and its role in treatment of osteoporosis. Med Sci Monit. 2016;22:226–233. doi: 10.12659/MSM.897044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wegner M. From head to toes: the multiple facets of Sox proteins. Nucleic Acids Res. 1999;27:1409–1420. doi: 10.1093/nar/27.6.1409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wegner M. All purpose Sox: The many roles of Sox proteins in gene expression. Int J Biochem Cell Biol. 2010;42:381–390. doi: 10.1016/j.biocel.2009.07.006. [DOI] [PubMed] [Google Scholar]
- Xu X., Jia X., Mo L., Liu C., Zheng L., Yuan Q., Zhou X. Intestinal microbiota: a potential target for the treatment of postmenopausal osteoporosis. Bone Res. 2017;5:17046. doi: 10.1038/boneres.2017.46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang N., Wang G., Hu C., Shi Y., Liao L., Shi S., Cai Y., Cheng S., Wang X., Liu Y., et al. Tumor necrosis factor alpha suppresses the mesenchymal stem cell osteogenesis promoter miR-21 in estrogen deficiency-induced osteoporosis. J Bone Miner Res. 2013;28:559–573. doi: 10.1002/jbmr.1798. [DOI] [PubMed] [Google Scholar]
- Yao W., Guan M., Jia J., Dai W., Lay Y.A., Amugongo S., Liu R., Olivos D., Saunders M., Lam K.S., et al. Reversing bone loss by directing mesenchymal stem cells to bone. Stem Cells. 2013;31:2003–2014. doi: 10.1002/stem.1461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao J.W., Gao Z.L., Mei H., Li Y.L., Wang Y. Differentiation of human mesenchymal stem cells: the potential mechanism for estrogen-induced preferential osteoblast versus adipocyte differentiation. Am J Med Sci. 2011;341:460–468. doi: 10.1097/MAJ.0b013e31820865d5. [DOI] [PubMed] [Google Scholar]