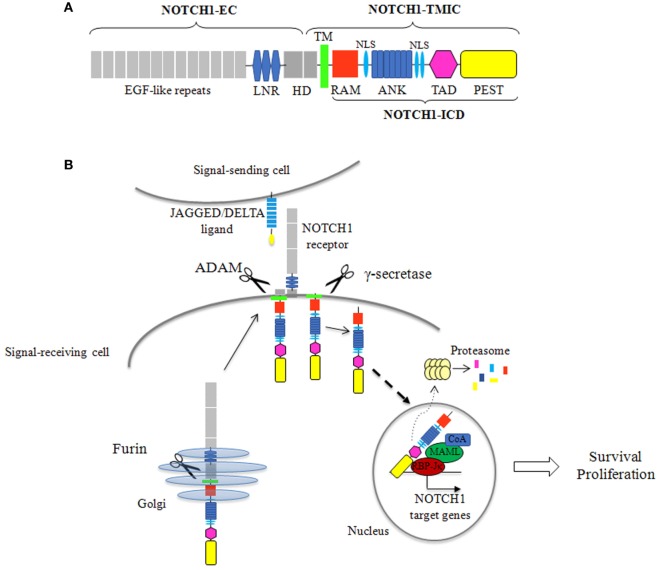
Figure 1.
NOTCH1 protein structure and signaling activation. (A) The mature NOTCH1 receptor is a heterodimer composed of an extracellular subunit (NOTCH1-EC) and a transmembrane and intracellular subunit (NOTCH1-TMIC). The NOTCH1-EC includes epidermal growth factor (EGF)-like repeats, involved in ligand binding, three LIN-12/NOTCH repeats (LNR), which prevent receptor activation in the absence of ligands, and the heterodimerization domain (HD) involved in non-covalent interactions between the NOTCH1-EC and NOTCH1-TMIC. The NOTCH1-TMIC consists of the transmembrane domain (TM) and the intracellular domain (ICD) (NOTCH1-ICD). NOTCH1-ICD comprises an RBPJ-associated molecule (RAM) domain, seven ankyrin (ANK) repeats, nuclear localization signals (NLS), a transactivation domain (TAD), and a PEST domain, which is involved in proteasomal degradation of active NOTCH1-ICD. (B) Newly synthesized NOTCH1 precursor is cleaved by a furin-like convertase (Furin) in the Golgi apparatus to generate the mature receptor. NOTCH1 signaling initiates when a JAGGED or DELTA ligand expressed on a signal sending cell interacts with NOTCH1 on a signal receiving cell. This interaction triggers two sequential cleavages of NOTCH1: the first, by an a disintegrin and metalloproteinase (ADAM) metalloproteinase, generates the substrate for the second cleavage by γ-secretase, which releases the active NOTCH1-ICD. NOTCH1-ICD translocates to the nucleus where it forms a transcriptional activation complex by interacting with the transcription factor CSL/RBP-Jk, mastermind-like proteins, and others coactivators (CoA), leading to the expression of NOTCH1 target genes. In physiological conditions, NOTCH1 signal attenuation is mediated by ubiquitination and proteasomal degradation of NOTCH1-ICD.