Figure 1. Structural model of PE38 and illustrations of the different αTac domain II mutants.
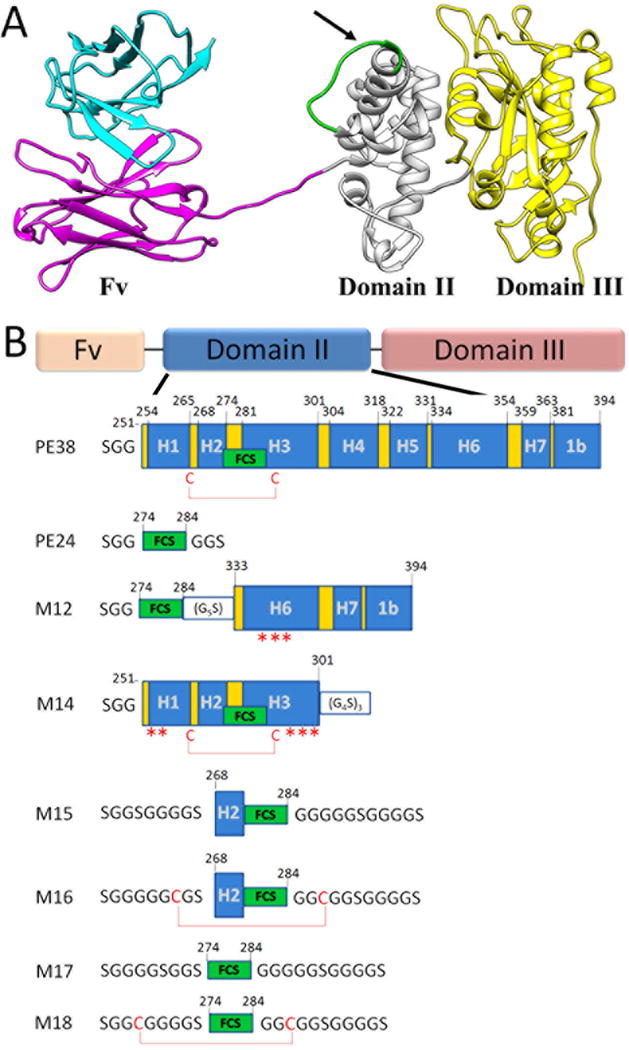
(A) αTac-dsFv-PE38 is composed of an αTac dsFv targeting moiety followed by the PE38 toxin comprising domains II and III. Note the FCS marked in green within PE domain II. The arrow signifies the cleavage site. (B) Illustrations of the rationally designed domain II truncation mutants. PE domain II is divided into seven helixes, designated H1-H7, with helixes H1, H3, H4, H5 and H6 bundled around a hydrophobic core. The FCS (residues 274-284, “RHRQPRGWEQL”) naturally resides in helixes H2 and H3 of domain II, and is constrained by a disulfide bond formed between Cys265 and Cys287. Mutants were designed based upon the crystal structure of the full PE38 (PDB ID: 1IKQ). Helixes are marked H1-H7, the FCS (“RHRQPRGWEQL”) is marked as a green box, disulfide bonds are shown as red lines, while asterisks denote solubilizing point mutations (hydrophobic to polar). The numbering system is based upon the mature PE38.
