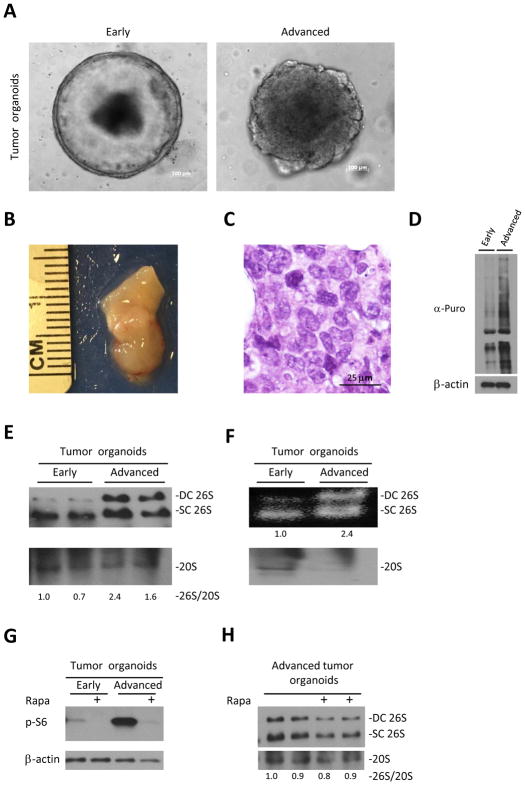
Figure 4. Progression of the tumor organoids is associated with increase in assembly of 26S.
A. Representative images of early and advanced organoids originating from large bowel tumors of APCmin/+ mice. B. Representative picture of xenograft deriving from advanced tumor organoids, 6 weeks after injection to NSG mice. C. H&E staining of advanced tumor organoids xenograft reveals cells with different nuclear size and shape, prominent nucleoli with almost no gland formation characteristic of malignant cells. D. Large bowel early and advanced tumor organoids of APCmin/+ mice were cultured in the presence of 4 uM Puromycin for 30 min. Puromycin incorporation, detected by anti-puromycin antibody, shows increase in translation with tumor progression.
E. Native gel electrophoresis of tumor organoids shown in A. demonstrates increase of 26S assembly in advanced tumor organoids. 26S and 20S were detected by anti-RPT1 and anti-Alpha7 antibodies respectively. F. In gel suc-LLVY-AMC activity assay (no SDS) of tumor organoids shown in A. shows increased in the amount of active 26S proteasomes in advanced tumor organoids at expense of unassembled 20S. G. Western blot of organoids cultured with/without 300nM of Rapamycin (Rapa) for 6 hours shows activation of mTORC1 in advanced organoids as expressed in the levels of pS6 H. Native gel electrophoresis of advanced tumor organoids cultured with/without 300nM of Rapamycin (Rapa) for 6 hours demonstrates no effect of mTORC1 inhibition on 26S assembly. DC 26S – Double capped 26S, SC 26S – Single capped 26S. Densitometry was performed using ImgeJ. 26S proteasome assembly status(26S/20S) is reported as the relative intensity of 26S (DC+SC) divided by intensity of 20S.