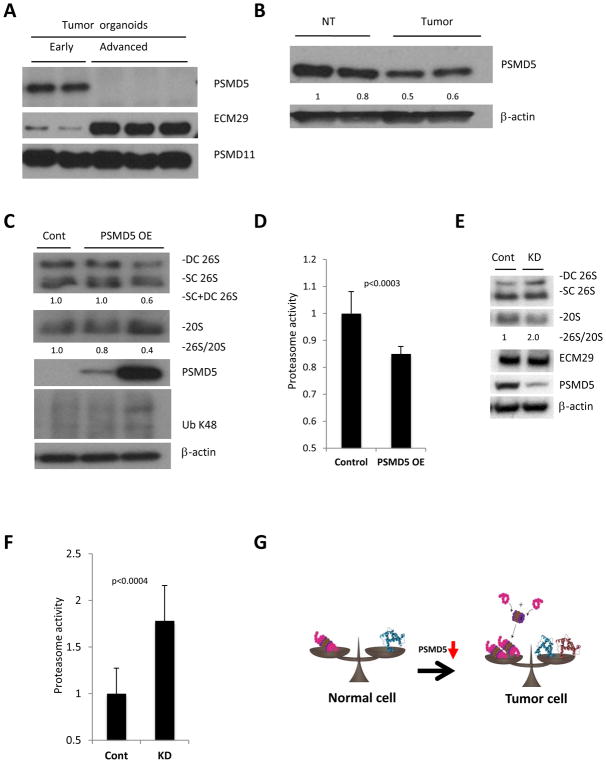
Figure 5. PSMD5 is a possible mediator of enhanced proteasome assembly in tumors with high protein turnover.
A. Western blot analysis of potential regulators of 26S assembly identified in the RNA-seq, showing significant reduction of PSMD5 and elevation of ECM29 in advanced organoids, with no change in PSMD11. B. Western blot of large bowel normal tissue and tumors from APCmin/+ mice shows reduced levels of PSMD5 in intestinal tumors. C. Western blot analysis following native gel electrophoresis (two upper panels) and SDS-Page (3 lower panels) of advanced organoids. Over-expression of PSMD5 leads to reduced assembly of 26S proteasomes and accumulation of poly-ubiquitinated proteins. D. Proteasome activity of cell lysates of advanced tumor organoids overexpressing PSMD5 and their controls. Values obtained for controls were set as 1. The error bars represent the standard deviation from triplicate measurements of 3 biological repetitions. Statistical analysis was performed with t-test. E. Western blot analysis following native gel electrophoresis (two upper panels) and SDS-Page (3 lower panels) of HT-29 cells transfected with control siRNA (Cont) or PSMD5 siRNA (KD). F. Proteasome activity of HT-29 cells transfected with control siRNA (Cont) or PSMD5 siRNA (KD). Values obtained for controls were set as 1. The error bars represent the standard deviation from average of 12 biological replicates. Statistical analysis was performed with a t-test. G. A model of enhanced 26S proteasome assembly in cancer cells. Upon malignant transformation tumor cells start to synthesize more protein. In order to degrade these excess proteins, tumor cells stimulate assembly of 26S proteasomes by inhibition of PSMD5 expression. DC 26S – Double capped 26S, SC 26S – Single capped 26S. 26S and 20S were detected by anti-RPT1 and anti-Alpha7 antibodies, respectively. Densitometry was performed using ImgeJ. 26S proteasome assembly status (26S/20S) is reported as the relative intensity of 26S (DC+SC) divided by intensity of 20S.