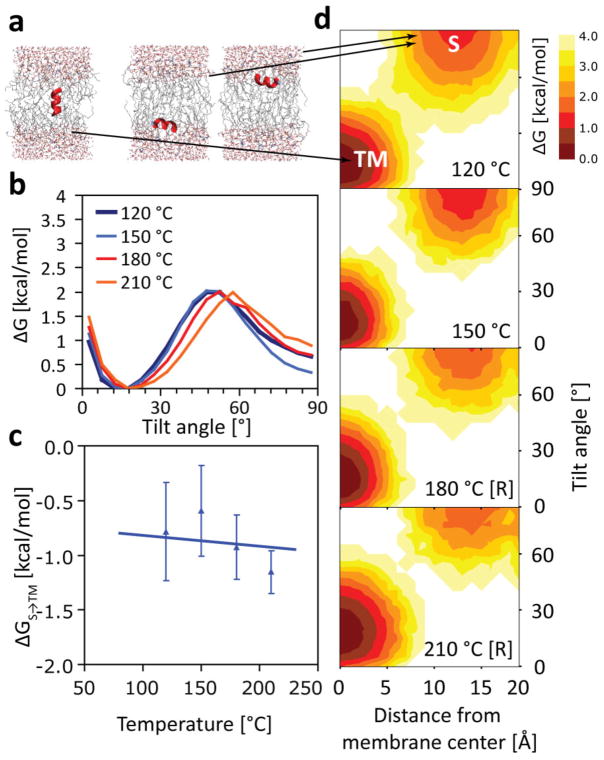
Figure 2.
(a) Temperature independence of hydrophobic S→TM partitioning for the L8 peptide in equilibrium simulations using the CHARMM36 force field. No pronounced temperature effect is visible over the range 120–210 °C. Each simulation plotted here is at least 5-μs long, so one-dimensional free energy profiles (b), and the overall transfer free energy ΔGS→TM (c), are well converged. Note that barrier in (b) is not a true transition state, which is undersampled in our equilibrium simulations. Simulations using helical restraints are denoted with [R]. No unfolding is observed otherwise, and both unrestrained and restrained simulations yield the same ΔGS→TM ≈ −0.9 ± 0.2 kcal/mol (c). Two-dimensional free energy profiles (as a function of membrane insertion and tilt angle) reveal almost no variation with temperature (d).