FIG 2 .
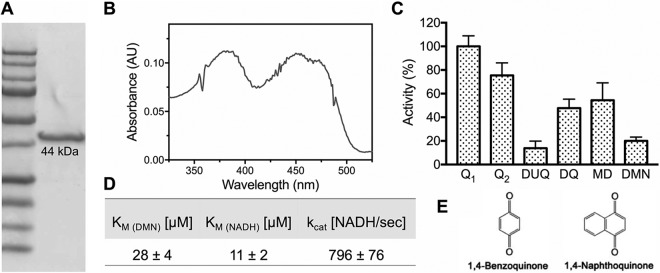
The isolated enzyme from S. agalactiae is a highly active NDH-2. (A) SDS-PAGE of ~5 µg of isolated protein. (B) UV-Vis spectrum of the flavin. (C) Enzyme activity was measured with different soluble quinone analogues. Data are expressed as average ± standard deviation (SD) from three independent experiments. Q1, ubiquinone-1; Q2, ubiquinone-2; DUQ, decylubiquinone; DQ, duroquinone; MD, menadione; DMN, 2,3-dimethyl-1,4-naphthoquinone. One hundred percent activity corresponds to 4,190 NADH s−1 observed in the presence of Q1. (D) Turnover number (kcat) and affinity (Km) for the enyzme’s substrates were determined. Data are expressed as average ± SD from three independent experiments. (E) Core structures for benzoquinones (Q1, Q2, DUQ, and DQ) and naphthoquinones (MD and DMN).