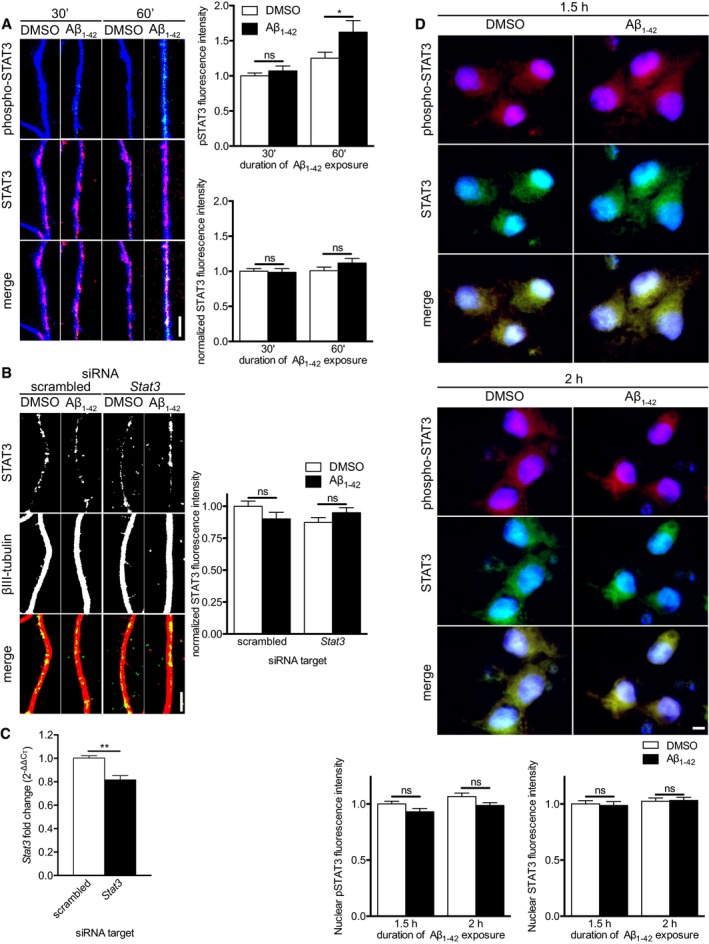
Hippocampal neurons were cultured in microfluidic chambers for 11–12 DIV, and axons were treated with vehicle or Aβ1–42 for 30 or 60 min. Axons were immunostained for phospho‐STAT3 (Tyr705), STAT3, and βIII‐tubulin. Mean ± SEM of 39–40 optical fields (n = 8 independently performed experiments). *P < 0.05; ns, not significant; two‐way ANOVA with Bonferroni's multiple comparisons test.
Hippocampal neurons were cultured in microfluidic chambers for 11–12 DIV, and axons were transfected with scrambled or Stat3‐targeting siRNA. 24 h after transfection, axons were pre‐treated with ciliobrevin A for 45 min before addition of vehicle or Aβ1–42 for 1 h. Axons were immunostained for STAT3 and β‐III‐tubulin. Mean ± SEM of 40 optical fields per condition (n = 8 independently performed experiments). ns, not significant; Holm–Sidak multiple t‐tests.
Dissociated hippocampal neurons were cultured for 11–12 DIV. Neurons were transfected with scrambled or Stat3‐targeting siRNA. 48 h after transfection, neurons were lysed, and RNA was purified. Stat3 transcript levels were quantified using qRT–PCR. Mean ± SEM of six independently performed experiments (n = 6) each with triplicate qRT–PCR measurements. **P < 0.01; unpaired t‐test.
Hippocampal neurons were cultured in microfluidic chambers for 11–12 DIV and axons were treated with vehicle or Aβ1–42 for 1.5 or 2 h. Neurons were immunostained for pSTAT3 and STAT3, and nuclei were stained with DAPI. Mean ± SEM for 39–50 optical fields (n = 8–10 independently performed experiments). ns, not significant; two‐way ANOVA with Bonferroni's multiple comparisons test.
Data information: Scale bars, 5 μm.