Abstract
Background
Late-life depression patients are at a high risk of developing Alzheimer’s disease, and diminished olfactory identification is an indicator in early screening for Alzheimer’s disease in the elderly. However, whether diminished olfactory identification is associated with risk of developing Alzheimer’s disease in late-life depression patients remains unclear.
Methods
One hundred and twenty-five late-life depression patients, 50 Alzheimer’s disease patients, and 60 normal controls were continuously recruited. The participants underwent a clinical evaluation, olfactory test, neuropsychological assessment, and neuroimaging assessment.
Results
The olfactory identification impairment in late-life depression patients was milder than that in Alzheimer’s disease patients. Diminished olfactory identification was significantly correlated with worse cognitive performance (global function, memory language, executive function, and attention) and reduced grey matter volume (olfactory bulb and hippocampus) in the late-life depression patients. According to a multiple linear regression analysis, olfactory identification was significantly associated with the memory scores in late-life depression group (B=1.623, P<.001). The late-life depression with olfactory identification impairment group had worse cognitive performance (global, memory, language, and executive function) and more structural abnormalities in Alzheimer’s disease-related regions than the late-life depression without olfactory identification impairment group, and global cognitive function and logical memory in the late-life depression without olfactory identification impairment group was intact. Reduced volume observed in many areas (hippocampus, precuneus, etc.) in the Alzheimer’s disease group was also observed in late-life depression with olfactory identification impairment group but not in the late-life depression without olfactory identification impairment group.
Conclusion
The patterns of cognitive impairment and structural abnormalities in late-life depression with olfactory identification impairment patients were similar to those in Alzheimer’s disease; olfactory identification may help identify late-life depression patients who are at a high risk of developing Alzheimer’s disease.
Keywords: late-life depression, olfactory, Alzheimer’s disease, neuropsychology, neuroimaging
Significance Statement
Individuals with late-life depression (LLD) are at a high risk of developing Alzheimer’s disease (AD), and diminished olfactory identification (OI) serves as an effective biomarker in predicting AD in older adults. However, whether diminished OI is associated with the risk of developing AD in LLD patients remains unclear. We found that diminished OI is correlated with poor cognitive performance (particularly memory) and brain atrophy (olfactory bulb and hippocampus) in LLD patients. The LLD with OI impairment group had worse cognitive performance and more structural abnormalities in AD-related regions than the LLD without OI impairment group, and the patterns of structural abnormalities in the LLD with OI impairment patients were similar to those observed in the AD patients. The strong associations among diminished OI, cognitive impairment, and atrophy in AD-related regions in the LLD patients and the similarity in cognitive impairment and structural abnormalities between the LLD with OI impairment patients and AD patients suggest that OI may serve as an indicator for identifying LLD patients at a high risk of developing AD.
Introduction
Late-life depression (LLD) is among the most common disabling mental illnesses in older people and affects 3.5 to 7.5% of the geriatric population (Weyerer et al., 2005). LLD patients are considered at a high risk of developing dementia (Kaup et al., 2016; Mirza et al., 2016), and LLD may share common mechanisms with Alzheimer’s disease (AD), such as alterations in glucocorticoid steroids, hippocampal atrophy, vascular disease, deficits in brain-derived neurotrophic factors, inflammatory changes, and increased deposition of β-amyloid plaques (Butters et al., 2008; Byers and Yaffe, 2011; Wang and Dan, 2014). Whether LLD is an early manifestation or a risk factor of dementia remains controversial; however, the early identification of LLD patients who are most likely to develop AD could be advantageous for timely intervention. Comprehensive assessments of cognitive function may contribute to this early screening process, and LLD patients with poor cognitive performance (particularly memory deficits) exhibit more structural abnormalities in AD-related regions, more functional and white matter network abnormalities, a high amyloid load with hypermetabolism, increased cognitive decline, and higher rates of conversion to AD (Lee et al., 2012; Brendel et al., 2015; Li et al., 2015; Mai et al., 2017), suggesting that LLD patients with cognitive impairment may have a higher risk of developing AD. However, suboptimal effort may be common in LLD patients and lead to bias in neuropsychological assessments (Benitez et al., 2011); thus, early screening requiring less time, cost and subjective efforts may be more desirable for differentiating individuals at a high risk of developing AD from LLD patients.
Recently, diminished olfactory identification (OI) has been used as a supplemental assessment for the early detection of AD and an effective biomarker of AD pathology due to its advantages of being simple, cost-effective, and noninvasive (Laske et al., 2015; Roberts et al., 2016; Lafaillemagnan et al., 2017). OI, which is the ability to identify and denominate specific odors, depends on several cognitive processes, such as semantic memory access, denomination capacities, and comprehension of instructions (Rahayel et al., 2012; Roberts et al., 2016). Therefore, OI impairment is considered to reflect the extent of cognitive impairment and brain malfunction in older individuals. In cross-sectional studies, individuals with diminished OI exhibited worse cognitive performance (memory, execution function, and language) (Roberts et al., 2016), reduced hippocampal volume (HV) and olfactory bulb volume (OBV) (Thomann et al., 2009), a thinner entorhinal cortex, increased cortical amyloid burden (Growdon et al., 2015), reduced blood flow in the frontotemporal lobe (Wang et al., 2010), lower ratios of CSF t-tau and P181-tau to Aβ1–42 (Lafaillemagnan et al., 2017), and a high proportion of these patients were APOE ε4 carriers (Dhilla et al., 2016). In a longitudinal study, OI impairment predicted a faster cognitive decline and higher rate of conversion to AD in amnestic mild cognitive impairment (aMCI) patients and elderly controls (Roberts et al., 2016), particularly in combination with neuropsychological assessments and neuroimaging evaluations (Devanand et al., 2008; Lojkowska et al., 2011).
However, whether diminished OI could contribute to identifying LLD patients at a high risk of developing AD remains unclear, because studies specifically focusing on OI in LLD patients are lacking, and previous studies investigating olfactory function in depression patients did not exclusively focus on older adults. It has been repeatedly reported that it was olfactory threshold (OT) rather than OI that was significantly impaired in patients with depression (Naudin and Atanasova, 2014; Khil et al., 2016; Croy and Hummel, 2017). In addition, OI impairment is only pronounced in depression patients with first-time high symptom severity and severe disease courses, and the prevalence of OI impairment in depression patients is similar to that in normal controls (approximately 15%) (Khil et al., 2016). The patterns of olfactory impairment differ between AD and depression patients, and OI deficits may be significant in AD patients but not in depression patients (Naudin and Atanasova, 2014); thus, previous studies involving small sample sizes have successfully differentiated AD patients from depression patients using OI tests. At a cut-off score of 10/11 on the Sniffin’ Sticks identification test, the specificity and sensitivity of OI impairment in distinguishing AD patients from depression patients is 95% and 100%, respectively (Solomon et al., 1998), and these rates are similar using the Pocket Smell Test (sensitivity=80% and specificity=100%) (Pentzek et al., 2007).
Because LLD patients with cognitive impairment are more likely to convert to AD, and OI impairment is associated with cognitive impairment (particularly memory deficit) and the risk of developing AD, diminished OI may contribute to identifying prodromal AD in LLD patients, and LLD patients with OI impairment (LLD-OII) may be at a high risk of developing AD. Therefore, in the present study, we aimed to determine whether (1) LLD patients suffer from significant OI impairment, (2) diminished OI is associated with the cognitive impairment and reduced grey matter volume in LLD patients, (3) LLD-OII patients have worse cognitive impairment and more structural abnormalities than LLD patients without OI impairment (LLD-NOII), and (4) the neuropsychological and neuroimaging characteristics of LLD-OII patients are similar to those of AD patients.
METHODS
Participants
One hundred and twenty-five LLD patients and 50 AD patients were continuously recruited from the Affiliated Brain Hospital of Guangzhou Medical University (Guangzhou Huiai Hospital), and 60 normal controls (NCs) were recruited from the community in Guangzhou between May 2016 and July 2017. All subjects in our study were from the Chinese Han population. All participants or their legal guardians provided written informed consent to participate in the study. This study was approved by the ethics committees of The Affiliated Brain Hospital of Guangzhou Medical University (Guangzhou Huiai Hospital).
The inclusion criteria for the LLD group were as follows: (1) patients >55 years of age; (2) patients who met the criteria for major depression disorder according to the DSM-IV after the age of 55; and (3) patients whose clinical stage and diagnosis were confirmed by a trained physician at our hospital. The following exclusion criteria were applied: (1) a history of another major psychiatric disorder, such as bipolar disorder or schizophrenia, (2) a family history of schizophrenia or bipolar disorder, (3) physical illness that may induce emotion abnormalities, such as anaemia or hypothyroidism, (4) neurological disease, such as brain tumor or stroke, and (5) current or previous psychotic symptoms. AD was diagnosed according to the NINCDS-ADRDA criteria by 2 trained neurologists (Mckhann et al., 1984). The LLD and AD diagnoses were the primary diagnoses. A system interview was performed to collect participants’ demographic data and information regarding comorbidities, personal history, family history, medication history, surgical history, and allergy history, and physical and neurological examinations were performed.
Neuropsychological Assessments
After the participants underwent standard clinical assessments, they were interviewed by neuropsychologists to assess their global cognitive functioning using the Mini-mental State Examination (MMSE) and depressive state using the 17-item Hamilton Depression Rating Scale (HAMD-17). In addition, the participants in the NC and LLD groups underwent comprehensive neuropsychological tests to assess their performance in the following 5 cognitive domains: memory (the Logical Memory Test [LMT]), auditory (the Verbal Learning Test [AVLT]), language (the Boston Naming Test [BNT], and the Verbal Fluency Test [VFT]), executive function (the Stroop Colour and Word Test [Stroop] and the Trail-Making Test [TMT]), attention (the Symbol-Digit Modality Test [SDMT] and the Digit Span Test [DST]), and visuospatial skills (the Clock Drawing Test 4 [CDT4] and the Rey–Osterrieth complex figure [ROCF] test). The raw tests scores were adjusted using normative data, summed, and scaled to compute the domain z scores (Ivnik et al., 1992; Roberts et al., 2016).
Olfactory Assessments
The olfactory function, including OI and OT, was assessed using the Sniffin’ Sticks Screen 16 test (Hummel et al., 1997). The subjects completed a questionnaire surveying factors that may influence olfactory function (i.e., history of nasal trauma and surgery, history of radiation or chemotherapy, difficulty breathing through one side of the nose, etc). Subjects were excluded if they had an active upper respiratory/sinus infection or respiratory distress at the time of testing, congenital or traumatic anosmia, known nasal polyps or tumor, current or recent (prior 6 months) smoking status, and alcohol or substance dependence (Hummel et al., 1997). All olfactory assessments were performed in a quiet, odorless, ventilated room at the Affiliated Brain Hospital of Guangzhou Medical University. All participants underwent the OT and OI tests following neuropsychological assessments.
Neuroimaging Assessment
Forty-five LLD patients, 20 AD patients, and 25 NC subjects volunteered to undergo MRI after the neuropsychological assessments and olfactory tests. All participants were ethnically Chinese Han and right-handed.
MRI Data Acquisition
The Philips 3.0 T MR system at the Affiliated Brain Hospital of Guangzhou Medical University was used to acquire the imaging data. For each participant, an anatomical image was obtained using a sagittal 3D gradient-echo T1-weighted sequence (TR=8.2 ms, TED=3.8 ms, TI=1100 ms, flip angle=8°, 188 slices, slice thickness=1 mm, Gap=0 mm, matrix=256x256, and inversion time=0).
Image Processing
The MR data were preprocessed using the toolboxes in Statistical Parametric Mapping 12 (SPM 12, University College London) (Tzourio-Mazoyer et al., 2002). Briefly, each T1 image was segmented into the cerebrospinal fluid, white matter, and grey matter and then normalized to the Montreal Neurological Institute template. A Gaussian kernel filter of 8x8x8 mm3 was used to smooth the modulated image.
VBM Analysis
A general linear model was performed to investigate the smoothed modulated grey matter images using SPM 12. An ANCOVA was performed to analyze the voxel-wise grey matter volume (Ashburner and Friston, 2000) differences among the groups, and the Least Significant Difference (LSD) posthoc test was used to perform the multiple comparisons; the covariates entered in the model included the total brain volume (=total white matter volume + total grey matter volume), age, sex, and years of education. This correction was confined in a grey matter mask using SPM 12 and adjusted using false discovery rate corrections (P<.05). The mean left and right hippocampal grey matter volumes (HVs) were then extracted from the regions of interest using the anatomical automatic labelling brain atlas (Tzourio-Mazoyer et al., 2002).
OBV
The OBV was calculated by planimetric manual contouring, and all surfaces were added and multiplied by 1 (1-mm slice thickness) to obtain the volume in mm2. Details regarding the OBV assessment are provided by Yousem et al. (Yousem et al., 1998), and this procedure has been used in many studies investigating the OBV (Negoias et al., 2010, 2016; Croy et al., 2013; Hummel et al., 2013). The OBV assessments were performed by the same experimenter who was blind to the participants’ conditions.
Statistic
The Statistical Package for the Social Sciences version 22.0 (IBM SPSS 22.0) was used to perform the statistical analyses. Chlorpromazine equivalent doses were used to unify the doses of different medicines (Woods, 2003), and fluoxetine equivalent doses were used to unify the doses of different antidepressants (Yu et al., 2015). The differences in the demographic and clinical variables among the groups were evaluated by performing a χ2 analysis, 1-way ANOVA, and Kruskal-Wallis nonparametric ANOVA as needed, and LSD posthoc tests were used to perform the multiple comparisons. Partial correlation analyses were performed to analyze the associations between the olfactory function and cognitive scores in the LLD patients; the control variables included age, sex, years of education, and HAMD scores. The associations between olfactory function and cognitive z scores in the LLD patients were analyzed by performing a multiple linear regression analysis. Partial correlation analyses were performed to analyze the associations among the behavioral indexes (OI and MMSE) and neuroimaging indexes (bilateral OBV and HV); the control variables included age, sex, and years of education. A score <10 indicated the presence of OI impairment (Hummel et al., 2001), and the LLD patients were divided into the following 2 groups: LLD-OII and LLD-NOII. An ANCOVA was performed to compare the cognitive scores among the NC without olfactory identification impairment (NC-NOII), LLD-OII, LLD-NOII, and AD; the control variables included age, sex, years of education, and HAMD scores. An ANCOVA was performed to compare the OBV among the 4 groups, and the control variables included age, sex, years of education, and total brain volume. LSD posthoc tests were used to perform the multiple comparisons.
Results
Demographic Data
The demographic data of the different groups are listed in Table 1. The NC group had significantly higher scores (MMSE, OI, and OT), and the AD group had significant lower scores (MMSE, OI, and OT) than the LLD group (P<.05). No significant difference was observed in the OT (t=1.001, P=.318) and olfactory identification (t=1.029, P=.305) between the males and females. No significant correlation was found between the olfactory function and chlorpromazine equivalent doses and between the olfactory function and fluoxetine equivalent doses in all participants (P>.05).
Table 1.
Demographic and Clinical Data of All Participants
| NC (n=60) | LLD (n=125) | AD (n=50) | F/χ 2 /Z | P | Posthoca | |
|---|---|---|---|---|---|---|
| Age | 65.4±7.3 | 66.7±6.2 | 71.9±9.9 | 11.702 | <.001 | - |
| Male/female | 24/36 | 29/96 | 22/28 | 9.535 | .009 | - |
| Years of education | 9.1±3.5 | 8.0±4.0 | 6.8±4.1 | 4.700 | .010 | - |
| HAMD | 1 (0, 3) | 7 (3, 15) | 5 (2, 9) | 69.010 | <.001 | - |
| MMSE | 26.8±1.9 | 22.7±5.3 | 12.4±5.1 | 133.389 | <.001 | A>B>C |
| OI | 11.8±1.7 | 9.9±2.7 | 5.8±1.8 | 88.795 | <.001 | A>B>C |
| OT | 7.6±2.5 | 6.0±2.7 | 4.6±1.8 | 17.417 | <.001 | A>B>C |
Abbreviations: AD, Alzheimer’s disease; HAMD, Hamilton Depression Rating Scale; LLD, late life depression; MMSE, Mini-mental State Examination; NC, normal control, OI, olfactory identification; OT, olfactory threshold.
aIn posthoc multiple comparisons, A means normal control group, B means LLD group, and C means AD group.
Correlation Analysis
OI was positively correlated with the MMSE, AVLT (immediate recall, short-term delayed recall, long-term delayed recall, and recognition), LMT (immediate recall and delayed recall), BNT, VFT, and digital span and negatively correlated with Stroop A in the LLD patients (P<.05) (see Table 2). No correlation was found between the OT and any cognitive scores (P>.05). According to the multiple linear regression analysis, OI was significantly correlated with the memory z scores in the LLD group (R2=0.242, B=1.623, P<.001, 95% CI was 1.056 to 2.190); however, no significant correlation was found between the OT scores and any cognitive z scores (P>.05).
Table 2.
Correlations between OI and Neuropsychological Test Scores in LLD Patients
| Cognition | Neuropsychological Test | r | P |
|---|---|---|---|
| Global | MMSE | 0.422 | <.001 |
| Memory | AVLT immediate recall | 0.401 | <.001 |
| short-term delayed Recall | 0.439 | <.001 | |
| long-term delayed Recall | 0.312 | .002 | |
| recognition | 0.332 | .012 | |
| LMT immediate recall | 0.382 | <.001 | |
| delayed Recall | 0.384 | <.001 | |
| Language | BNT | 0.314 | .002 |
| VFT | 0.322 | .001 | |
| Executive | TMT B | -0.128 | .211 |
| Stroop A | -0.223 | .028 | |
| Stroop SIE | -0.047 | .647 | |
| Visual-space | ROCF | 0.122 | .234 |
| CDT4 | -0.010 | .923 | |
| Attention | SMDT | 0.135 | .165 |
| Digital span | 0.220 | .030 |
Abbreviations: AVLT, auditory verbal learning; BNT, Boston Naming Test; CDT, Clock Drawing Test; DST, Digit Span Test; LMT, Logical Memory Test; ROCF, Rey-Osterrieth complex figure; SDMT, Symbol-Digit Modality Test; TMT, Trail-Making Test; VFT, Verbal Fluency Test.
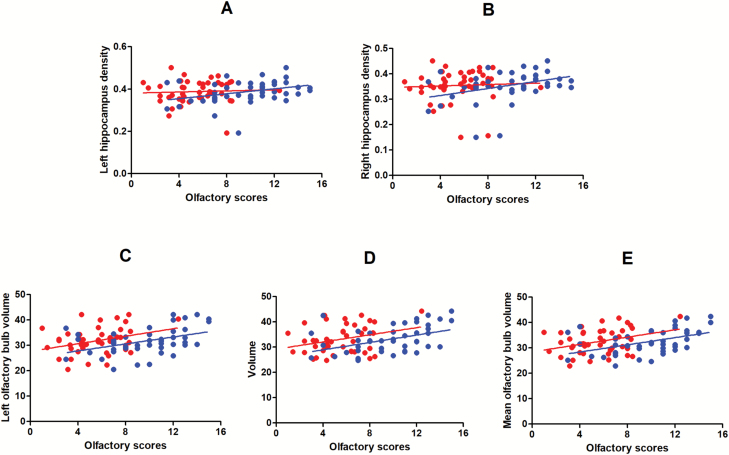
In the LLD group, the OI scores were positively correlated with the left OBV (r=0.348, P=.028), right OBV (r=0.371, P=.018), mean OBV (r=0.396, P=.011), left HV (r=0.316, P=.047), and right HV (r=0.324, P=.041). The OT scores were positively correlated with the mean OBV (r=0.328, P=.039). The MMSE scores were positively correlated with the left OBV (r=0.399, P=.011), right OBV (r=0.381, P=.015), mean OBV (r=0.429, P=.006), and left HV (r=0.398, P=.011) (see Figure 1).
Figure 1.
Partial correlation analysis of olfactory function and grey matter volume in patients with late life depression (LLD). Control variables included age, gender, and years of education. Blue represents olfactory identification (OI) and red represents olfactory threshold (OT). (A) OT (r=0.019, P=.906), OI (r= 0.316, P=.047). (B) OT (r=0.017, P=.919), OI (r= 0.324, P=.041); (C) OT (r=0.309, P=.052), OI (r=0.348, P=.028). (D) OT (r=0.288, P=.072), OI (r=0.371, P=.018). (E) OT (r=0.328, P=.039), OI (r=0.429, P=.006).
Comparison of Normal Controls, LLD Patients with or without OI Impairment, and AD Patients
The prevalence of OI impairment was 11.7% (n=7) in the NC group and 42.4% (n=53) in the LLD group (χ2=17.474, P<.001). The demographic data were as follows: NC-NOII group, male 19, female 34, age 65.3±7.3 years, and years of education 9.2±3.4; LLD-NOII group, male 14, female 58, age 66.4±6.0 years, years of education 8.4±3.9, and HAMD 7 (3, 15); and LLD-OII group, male 15, female 38, age 67.1±6.6 years, years of education 7.5±4.2, and HAMD 8 (4, 15). Both LLD groups exhibited overall cognitive impairment, but the LLD-OII group demonstrated worse cognitive performance (global, memory, language, executive function, and attention) than the LLD-NOII group (P<.05). A reduced OBV was found in the LLD-NOII, LLD-OII, and AD groups. Additional details are provided in Table 3.
Table 3.
Comparison among normal control, LLD with or without OI, and AD
| NC-NOII (n=53) | LLD-NOII (n=72) | LLD-OII (n=53) | AD (n=50) | χ 2 /Z/F | P | Posthoca | |
|---|---|---|---|---|---|---|---|
| OI | 12.2±1.4 | 11.8±1.4 | 7.3±1.8 | 5.8±1.8 | 171.827 | <.001 | A, B>C>D |
| OT | 7.9±2.4 | 6.3±2.4 | 5.3±2.6 | 4.6±1.8 | 8.774 | <.001 | A>B, C, D |
| HAMD | - | 7(3, 15) | 8(4, 15) | - | -0.689 | .491 | - |
| MMSE | 26.8±1.9 | 24.6±3.6 | 20.4±6.3 | 12.4±5.1 | 80.800 | <.001 | A, B>C>D |
| AVLT immediate recall | 21.3±4.1 | 18.1±5.4 | 13.9±5.6 | - | 14.180 | <.001 | A>B>C |
| Short-term delayed recall | 7.7±1.9 | 6.1±2.5 | 3.6±3.0 | - | 21.010 | <.001 | A>B>C |
| Long-term delayed recall | 7.0±2.3 | 5.2±2.8 | 3.2±3.2 | - | 12.505 | .012 | A>B>C |
| Recognition | 21.9±1.8 | 21.1±2.2 | 19.2±3.8 | - | 6.750 | .002 | A,B>C |
| LMT immediate recall | 5.7±2.6 | 4.6±2.8 | 3.0±2.4 | - | 6.738 | .002 | A,B>C |
| Delayed recall | 4.3±2.5 | 3.1±2.4 | 1.6±1.8 | - | 10.517 | <.001 | A,B>C |
| BNT | 22.7±2.6 | 20.5±3.9 | 18.2±4.9 | - | 10.493 | <.001 | A>B>C |
| VFT | 15.0±4.1 | 13.7±3.6 | 11.4±4.1 | - | 6.757 | .002 | A,B>C |
| TMTB | 60.8±28.6 | 80.8±33.7 | 88.2±36.8 | - | 4.831 | .009 | A<B,C |
| Stroop A | 27.7±5.8 | 33.0±8.1 | 37.4±10.6 | 9.734 | <.001 | A<B<C | |
| Stroop SIE | 51.3±26.7 | 59.5±33.0 | 60.4±29.9 | - | 0.276 | .759 | - |
| ROCF | 27.9±3.1 | 23.9±6.2 | 22.9±8.4 | - | 4.179 | .017 | A>B,C |
| CDT4 | 3.6±0.7 | 3.5±0.7 | 3.4±0.9 | - | 0.391 | .677 | - |
| SMDT | 37.6±11.0 | 26.6±9.7 | 25.0±11.8 | - | 7.236 | .001 | A>B,C |
| Digital span | 17.1±3.7 | 14.6±3.8 | 13.6±3.7 | - | 5.569 | .005 | A>B,C |
| Left OBVb | 37.40±4.01 | 33.12±4.91 | 29.98±5.04 | 27.27±4.10 | 13.386 | <.001 | A>B,C,D B>D |
| Right OBVb | 37.03±4.67 | 34.84±5.37 | 30.91±4.64 | 27.51±2.97 | 13.370 | <.001 | A,B>C>D |
| Mean OBVb | 37.35±4.04 | 33.98±4.61 | 30.44±4.43 | 27.39±3.22 | 16.022 | <.001 | A>B>C>D |
Abbreviations: AD, Alzheimer’s disease; AVLT, Auditory Verbal Learning; BNT, Boston Naming Test; CDT, Clock Drawing Test; DST, Digit Span Test; HAMD, Hamilton Depression Rating Scale; LLD-OII, late life depression with olfactory identification; LLD-NOII, late life depression without olfactory identification; LMT Logical Memory Test; MMSE, Mini-mental State Examination; NC-NOII, normal control without olfactory identification; OBV, olfactory bulb volume; OI, olfactory identification; OT, olfactory threshold; ROCF, Rey-Osterrieth complex figure, SDMT, Symbol-Digit Modality Test; TMT, Trail-Making Test; VFT Verbal Fluency Test.
aIn posthoc multiple comparisons, A means normal control without OI impairment group, B means LLD without OI impairment group, C means LLD with OI impairment group, D means AD group.
bComparison of OBV included 25 NC-NOII subjects, 25 LLD-NOII patients, 20 LLD-OII patients, and 20 AD patients.
VBM Analysis
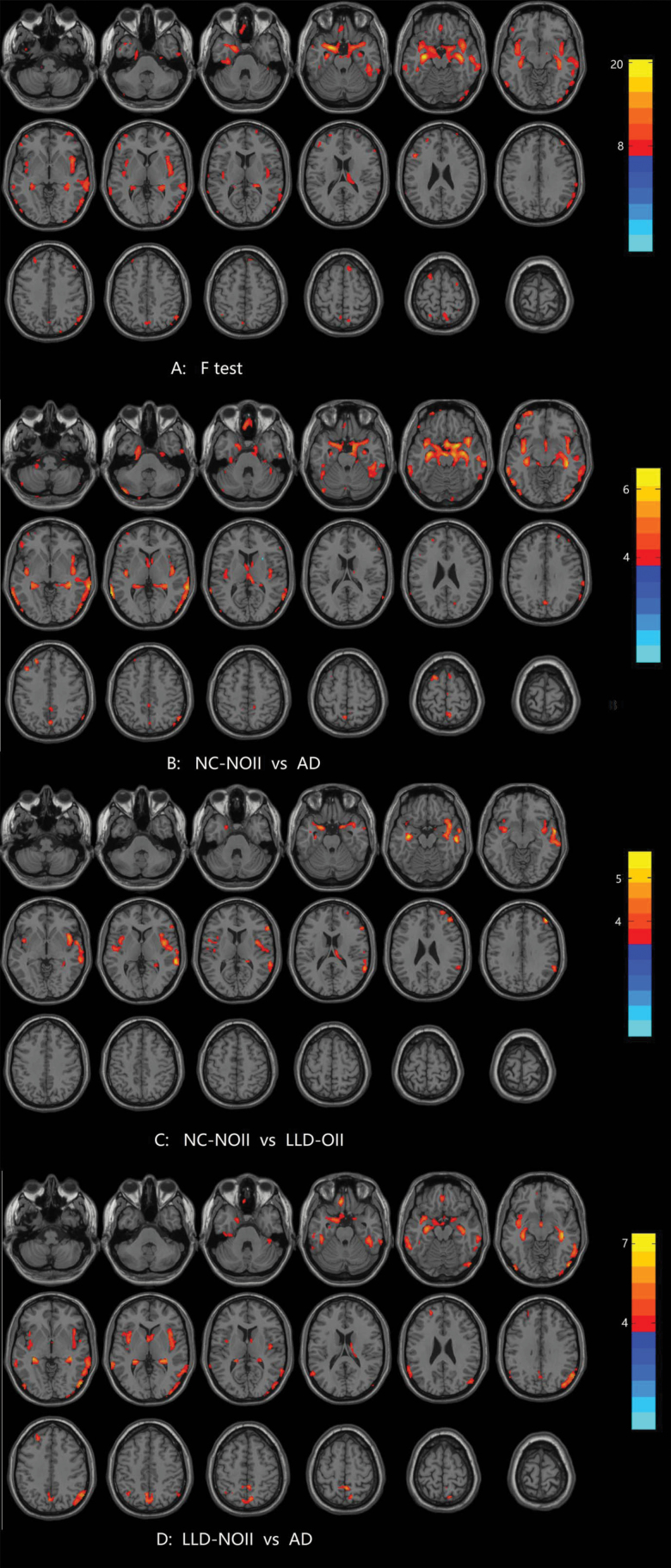
A significant difference was observed in many areas among the subjects in the NC-NOII, LLD-NOII, LLD-OII, and AD groups (see Table 4 and Figure 2A). Compared with the NC-NOII group, the AD group displayed significantly reduced grey matter volume in the bilateral hippocampus, bilateral parahippocampal gyrus, bilateral superior temporal gyrus, bilateral middle temporal gyrus, bilateral inferior temporal gyrus, bilateral amygdala, bilateral entorhinal cortex, right inferior occipital gyrus, left middle frontal gyrus, and left inferior frontal gyrus (see Figure 2B). Compared with the NC-NOII group, the LLD-OII group displayed diminished grey matter volume in the bilateral insula, bilateral hippocampus, bilateral parahippocampal gyrus, right superior temporal gyrus, right middle temporal gyrus, right inferior frontal gyrus, and right middle frontal gyrus (see Figure 2C). Additionally, compared with the LLD-NOII group, the AD group displayed significant grey matter volume reductions in the bilateral hippocampus, bilateral parahippocampal gyrus, bilateral insula, bilateral precuneus, left middle temporal gyrus, left superior temporal gyrus, left inferior temporal gyrus, left rectus gyrus, right middle temporal gyrus, right inferior temporal gyrus, right fusiform gyrus, right inferior occipital gyrus, right middle occipital gyrus, and right angular (see Figure 2D). No significant differences were observed between the NC-NOII and LLD-NOII groups, LLD-NOII and LLD-OII groups, and LLD-OII and AD groups.
Table 4.
Comparison of Grey Matter Volume among Normal Control without OI Impairment, LLD with/without OI Impairment, and AD
| Brain Area | MNI | ||||
|---|---|---|---|---|---|
| x | y | z | Cluster | F | |
| Left hippocampus | -32 | -12 | -16 | 5013 | 20.452 |
| Left middle temporal gyrus | 66 | -34 | 0 | 1601 | 12.814 |
| Right angular | 54 | -62 | 44 | 278 | 10.858 |
| Left lateral prefrontal cortex | -6 | 38 | -28 | 223 | 9.924 |
| Left inferior temporal gyrus | 48 | -34 | -22 | 180 | 11.668 |
| Left middle temporal gyrus | -67 | -44 | 8 | 126 | 8.969 |
| Left inferior frontal gyrus | -50 | 38 | 6 | 116 | 9.724 |
| Right precuneus | 12 | -64 | 64 | 113 | 9.224 |
| Left lateral inferior occipital cortex | -62 | -64 | -2 | 102 | 8.386 |
Abbreviation: MNI, Montreal Neurological Institute coordinate.
F test covariates included age, gender, years of education, and total brain volume. FDR correction, P<.05.
Figure 2.
Comparison of grey matter volume among normal control without olfactory identification (OI) impairment, and late life depression (LLD) with or without OI impairment and Alzheimer’s disease. ANCOVA, posthoc LSD test for multiple comparisons, covariates including total brain volume, age, gender, and years of education. This correction was confined within a grey matter mask and determined using false discovery rate (FDR) correction (P<.05). The color scale bar shows the logarithmic scale of P values (-log10). The closer to the yellow, the more significant is the difference between groups. Abbreviations: AD, Alzheimer’s disease; LLD-OII, late life depression with olfactory identification; LLD-NOII, late life depression without olfactory identification; NC-NOII, normal control without olfactory identification.
Discussion
This study is the first to focus on OI in LLD patients, and the AD risk in LLD patients was analyzed from the perspective of olfactory function for the first time. We primarily found 4 results. First, the LLD patients suffered from significant OI and OT impairment, and the AD patients had worse OI and OT than the LLD patients. Second, the diminished OI in the LLD patients was correlated with poor cognitive performance (particularly memory) and reduced grey matter volume (bilateral OBV and HV); the OT was positively correlated with the mean OBV; however, no correlations were observed between the OT and any cognitive scores in the LLD patients. Third, the LLD-OII group showed worse cognitive performance and more structural abnormalities than the LLD-NOII group, and no significant reduction in the grey matter volume was found in the LLD-NOII group. Finally, the reduced volume in many areas (hippocampus, precuneus, etc.) in the AD group was also observed in the LLD with OI impairment group but not in the LLD without OI impairment group.
In previous studies, the OT, but not OI, was significantly impaired (Naudin and Atanasova, 2014; Croy and Hummel, 2017; Khil et al., 2016) in depression patients, and the prevalence of OI impairment in depression patients (15.0%) was similar to that in the normal controls (15.3%) (Khil et al., 2016), suggesting that olfactory deficits in depression patients are likely caused by dysfunction in perceptual processes or primary olfactory cortex. In the present study, compared with the NC group, the LLD patients suffered from significant OT and OI impairment, and the prevalence of OI impairment in the LLD patients (41.6%) was significant higher than that in the NC subjects (10.2%). The increased prevalence of OI impairment in the depression patients in our study may be partially due to ageing, because all participants in our study were older, and OI deterioration may begin at the age of 50 (Zhang and Wang, 2017). However, because the prevalence of OI impairment in the NC subjects in this study was similar to that reported in a previous study, ageing may have only a limited contribution to the increased prevalence observed among the LLD patients. Therefore, the olfactory deficit in LLD patients may result from malfunctions in perceptual processes in primary and high-order olfactory cortices.
The olfactory pathway is intertwined with the cognitive pathway, and many brain areas, such as the OB, hippocampus, amygdala, and orbitofrontal cortex, that are involved in OI processing are also damaged in AD patients (Naudin and Atanasova, 2014). The OB is considered the first central relay station of the olfactory system, and the importance of the OB is not limited to olfactory processing. Reduced OBV may not only reduce odor input and lead to diminished OT but could also possibly affect neurotransmitter concentrations and alter functioning in limbic and reward related areas, resulting in significant OI impairment and cognitive deficits (Naudin and Atanasova, 2014; Croy and Hummel, 2017). Moreover, OB atrophy occurs early in the disease process of AD and is correlated with global cognitive impairment, suggesting that the OBV may be advantageous for the early recognition of AD (Thomann et al., 2009). The hippocampus is an important part of the secondary olfactory cortex and is among the first brain areas to be damaged in AD; the degree of hippocampal atrophy may be associated with disease severity (Poulin et al., 2011). Therefore, the presence of AD pathology and dysfunction in the hippocampus may lead to an inability to store and retrieve memories of smell and correctly identify odorants (Growdon et al., 2015). Consistent with previous studies, we found that the reductions in the OBV and HV were correlated with diminished OI and poor cognitive performance in the LLD patients. Additionally, OI impairment was associated with worse cognitive performance (global cognitive function, language, executive function, and attention), particularly severe memory deficits, in the LLD patients. This finding is consistent with previous observations in normal elderly individuals and aMCI patients (Roberts et al., 2016), suggesting that diminished OI may play a similar role in LLD patients as in normal elderly individuals and aMCI patients. Moreover, the OT was positively correlated with the OBV, but not the HV, and no correlation was found between the OT and any cognitive scores because the OT supposedly reflects malfunctions at the primary processing level.
Diminished OI has been repeatedly shown to contribute to identifying individuals at a high risk of developing AD among normal older adults and aMCI patients (Devanand et al., 2008; Lojkowska et al., 2011). Because certain LLD patients may be prodromal AD patients, and diminished OI is strongly associated with cognitive impairment and high-order brain area dysfunction in LLD patients, diminished OI may play a similar role in LLD patients, which could be advantageous in identifying individuals at a high risk of developing AD. We hypothesize that LLD-OII patients, but not LLD-NOII patients, may be at a high risk of developing AD, and the following 3 observations support our hypothesis.
First, the LLD-OII patients exhibited worse cognitive performance than the LLD-NOII patients. Both the LLD-OII and LLD-NOII patients suffered from overall cognitive impairment (memory, language, executive function, attention, and visuospatial skills), which is consistent with the results of previous studies (Wang and Blazer, 2015). However, global cognitive function, logical memory, verbal fluency, and verbal recognition in the LLD-NOII group was intact, and the LLD-OII group exhibited worse performance in the MMSE scores, all memory scores, all language scores, and Stroop A scores than the LLD-NOII group. Memory deficits are typical symptoms of AD (Mckhann et al., 1984), and the MMSE, AVLT, and LMT have long been used in early AD screening (Ivnik et al., 1990; Tombaugh and Mcintyre, 1992; Dumont et al., 1999;). In addition, LLD patients with cognitive impairment (memory deficit) have been shown to be at a high risk of developing AD (Lee et al., 2012). Therefore, the similar patterns of cognitive impairment between the LLD-OII patients and AD patients indicates that LLD-OII patients may be more susceptible to developing dementia than LLD-NOII patients.
Second, the LLD-OII patients had more structural abnormalities than the LLD-NOII patients. A significantly decreased grey matter volume was found in the LLD-OII group compared with that in the NC-NOII group, and the decreases in the AD group were greater than those in the LLD-NOII group; however, no significant difference was found between the LLD-NOII and NC-NOII groups, LLD-OII and AD groups, and LLD-OII and LLD-NOII groups. Thus, more structural abnormalities were observed in the LLD-OII patients than in the LLD-NOII patients, suggesting that LLD-OII patients may be at a higher risk of developing dementia.
Third, the pattern of structural abnormalities in the LLD-OII patients was similar to that in the AD patients. Many damaged areas in the LLD-OII patients, such as the hippocampus, OB, and precuneus, were not only altered in the AD patients in the present study but have also been repeatedly reported in previous studies involving patients who were preferentially affected by AD (Yang et al., 2012). Additionally, many structural abnormalities are neurostructural predictors of AD; for instance, hippocampal atrophy is the most typical neuroimaging characteristic of AD and has been listed as a diagnostic criterion for AD (Mckhann et al., 1984), and a reduced OBV may contribute to the early diagnosis of AD (Thomann et al., 2009). Considering that the pattern of structural abnormalities in the LLD-OII patients was similar to that in the AD patients, LLD-OII patients, but not LLD-NOII patients, may suffer from early AD, the prodrome of which is depression.
The present study has a few limitations. First, our results relied on a cross-sectional analysis. Longitudinal studies are needed to track the progression of cognitive decline and conversion rate of AD in patients with LLD and verify whether OI impairment could serve as a biomarker for differentiating early AD from patients with LLD. Second, OI deficits, depression, and cognitive impairment may also be predictors of Parkinson’s Disease, Dementia with Lewy Bodies, and other neurodegenerative diseases (Doty, 2008). Future studies combining CSF biomarkers, positron emission tomography, and gene polymorphisms will be advantageous for addressing potential confounds. Third, although we excluded participants with a current smoking status, acute respiratory tract infection, history of nasal surgery, and other conditions that could affect olfaction, we were unable to exclude the influence of a history of smoking, allergies, medicine, or nasal diseases because these aspects are common in most participants. Finally, we analyzed only the grey matter alterations in the participants, and their neuroimaging characteristics may not have been fully elucidated; further studies using multi-model inference and network-based analyses are currently in progress.
In summary, the LLD patients suffered from significant OI impairment, and diminished OI was associated with cognitive impairment (particularly memory deficit) and reduced grey matter volume (OB and hippocampus). The LLD-OII patients had worse cognitive performance and more structural abnormalities than the LLD-NOII patients, and the neuropsychological and neuroimaging characteristics of the LLD-OII patients were similar to those of the AD patients, suggesting that LLD-OII patients may be at a high risk of developing AD. Longitudinal studies are currently ongoing to determine whether LLD-OII patients have a faster cognitive decline and higher rate of conversion to AD and whether a combination of OI and other predictors improves the sensitivity and specificity of predicting AD.
Acknowledgments
This work was supported by the National Natural Science Foundation of China (nos. 81571333 and 85711333), Guangzhou Municipal Pharmaceutical Health Science and Technology Project (nos. 20151A031003 and 20141A011044), Guangzhou Municipal Science and Technology Program (no. 1563000496), and Science and Technology Department of Guangdong Province major science and technology (no. 2016B010108003).
Statement of Interest
None.
References
- Ashburner J, Friston KJ(2000)Voxel-based morphometry–the methods. Neuroimage 11:805–821. [DOI] [PubMed] [Google Scholar]
- Benitez A, Horner MD, Bachman D(2011)Intact cognition in depressed elderly veterans providing adequate effort. Arch Clin Neuropsychol 26:184–193. [DOI] [PubMed] [Google Scholar]
- Brendel M, Pogarell O, Xiong G, Delker A, Bartenstein P, Rominger A, Alzheimer’s Disease Neuroimaging Initiative (2015)Depressive symptoms accelerate cognitive decline in amyloid-positive MCI patients. Eur J Nucl Med Mol Imaging 42:716–724. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Butters MA, Young JB, Lopez O, Aizenstein HJ, Mulsant BH, Reynolds CF 3rd, DeKosky ST, Becker JT(2008)Pathways linking late-life depression to persistent cognitive impairment and dementia. Dialogues Clin Neurosci 10:345–357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Byers AL, Yaffe K(2011)Depression and risk of developing dementia. Nat Rev Neurol 7:323–331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Croy I, Hummel T(2017)Olfaction as a marker for depression. J Neurol 264:631–638. [DOI] [PubMed] [Google Scholar]
- Croy I, Negoias S, Symmank A, Schellong J, Joraschky P, Hummel T(2013)Reduced olfactory bulb volume in adults with a history of childhood maltreatment. Chem Senses 38:679–684. [DOI] [PubMed] [Google Scholar]
- Devanand DP, Liu X, Tabert MH, Pradhaban G, Cuasay K, Bell K, de Leon MJ, Doty RL, Stern Y, Pelton GH(2008)Combining early markers strongly predicts conversion from mild cognitive impairment to alzheimer’s disease. Biol Psychiatry 64:871–879. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dhilla Albers A, Asafu-Adjei J, Delaney MK, Kelly KE, Gomez-Isla T, Blacker D, Johnson KA, Sperling RA, Hyman BT, Betensky RA, Hastings L, Albers MW(2016)Episodic memory of odors stratifies alzheimer biomarkers in normal elderly. Ann Neurol 80:846–857. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Doty RL.(2008)The olfactory vector hypothesis of neurodegenerative disease: is it viable?Ann Neurol 63:7–15. [DOI] [PubMed] [Google Scholar]
- Dumont R, Willis JO, Veizel K, Zibulsky J(1999)Wechsler Abbreviated Scale of Intelligence. Rehabil Couns Bull 3346–3346. [Google Scholar]
- Growdon ME, Schultz AP, Dagley AS, Amariglio RE, Hedden T, Rentz DM, Johnson KA, Sperling RA, Albers MW, Marshall GA(2015)Odor identification and alzheimer disease biomarkers in clinically normal elderly. Neurology 84:2153–2160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hayasaka Y, Purgato M, Magni LR, Ogawa Y, Takeshima N, Cipriani A, Barbui C, Leucht S, Furukawa TA(2015)Dose equivalents of antidepressants: evidence-based recommendations from randomized controlled trials. J Affect Disord 180:179–184. [DOI] [PubMed] [Google Scholar]
- Hummel T, Sekinger B, Wolf SR, Pauli E, Kobal G(1997)‘Sniffin’ sticks’: olfactory performance assessed by the combined testing of odor identification, odor discrimination and olfactory threshold. Chem Senses 22:39–52. [DOI] [PubMed] [Google Scholar]
- Hummel T, Konnerth CG, Rosenheim K, Kobal G(2001)Screening of olfactory function with a four-minute odor identification test: reliability, normative data, and investigations in patients with olfactory loss. Ann Otol Rhinol Laryngol 110:976–981. [DOI] [PubMed] [Google Scholar]
- Hummel T, Henkel S, Negoias S, Galván JR, Bogdanov V, Hopp P, Hallmeyer-Elgner S, Gerber J, Reuner U, Haehner A(2013)Olfactory bulb volume in patients with temporal lobe epilepsy. J Neurol 260:1004–1008. [DOI] [PubMed] [Google Scholar]
- Ivnik RJ, Malec JF, Tangalos EG, Petersen RC, Kokmen E, Kurland LT(1990)The Auditory-Verbal Learning Test (AVLT): norms for ages 55 years and older. Psychol Assess 2:304–312. [Google Scholar]
- Ivnik RJ, Malec JF, Smith GE, Tangalos EG, Petersen RC, Kokmen E, Kurland LT(1992)Mayo’s older americans normative studies: WAIS—R norms for ages 56 to 97. Clin Neuropsychol 6:83–104. [Google Scholar]
- Kaup AR, Byers AL, Falvey C, Simonsick EM, Satterfield S, Ayonayon HN, Smagula SF, Rubin SM, Yaffe K(2016)Trajectories of depressive symptoms in older adults and risk of dementia. JAMA Psychiatry 73:525–531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Khil L, Rahe C, Wellmann J, Baune BT, Wersching H, Berger K(2016)Association between major depressive disorder and odor identification impairment. J Affect Disord 203:332–338. [DOI] [PubMed] [Google Scholar]
- Lafaille-Magnan ME, Poirier J, Etienne P, Tremblay-Mercier J, Frenette J, Rosa-Neto P, Breitner JCS, PREVENT-AD Research Group (2017)Odor identification as a biomarker of preclinical AD in older adults at risk. Neurology 89:327–335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laske C, Sohrabi HR, Frost SM, López-de-Ipiña K, Garrard P, Buscema M, Dauwels J, Soekadar SR, Mueller S, Linnemann C, Bridenbaugh SA, Kanagasingam Y, Martins RN, O’Bryant SE(2015)Innovative diagnostic tools for early detection of alzheimer’s disease. Alzheimers Dement 11:561–578. [DOI] [PubMed] [Google Scholar]
- Lee GJ, Lu PH, Hua X, Lee S, Wu S, Nguyen K, Teng E, Leow AD, Jack CR Jr, Toga AW, Weiner MW, Bartzokis G, Thompson PM, Alzheimer’s Disease Neuroimaging Initiative (2012)Depressive symptoms in mild cognitive impairment predict greater atrophy in alzheimer’s disease-related regions. Biol Psychiatry 71:814–821. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li W, Ward BD, Liu X, Chen G, Jones JL, Antuono PG, Li SJ, Goveas JS(2015)Disrupted small world topology and modular organization of functional networks in late life depression with and without amnestic mild cognitive impairment. J Neurol Neurosurg Psychiatry 86:1097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lojkowska W, Sawicka B, Gugala M, Sienkiewicz-Jarosz H, Bochynska A, Scinska A, Korkosz A, Lojek E, Ryglewicz D(2011)Follow-up study of olfactory deficits, cognitive functions, and volume loss of medial temporal lobe structures in patients with mild cognitive impairment. Curr Alzheimer Res 8:689–698. [DOI] [PubMed] [Google Scholar]
- Mai N, Zhong X, Chen B, Peng Q, Wu Z, Zhang W, Ouyang C, Ning Y(2017)Weight rich-club analysis in the white matter network of late-life depression with memory deficits. Front Aging Neurosci 9:279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McKhann G, Drachman D, Folstein M, Katzman R, Price D, Stadlan EM(1984)Clinical diagnosis of alzheimer’s disease: report of the NINCDS-ADRDA work group under the auspices of department of health and human services task force on alzheimer’s disease. Neurology 34:939–944. [DOI] [PubMed] [Google Scholar]
- Mirza SS, Wolters FJ, Swanson SA, Koudstaal PJ, Hofman A, Tiemeier H, Ikram MA(2016)Ten-year trajectories of depressive symptoms and risk of dementia: a population-based study. Lancet Psychiatry 3:628–635. [DOI] [PubMed] [Google Scholar]
- Naudin M, Atanasova B(2014)Olfactory markers of depression and Alzheimer’s disease. Neurosci Biobehav Rev 45:262–270. [DOI] [PubMed] [Google Scholar]
- Negoias S, Croy I, Gerber J, Puschmann S, Petrowski K, Joraschky P, Hummel T(2010)Reduced olfactory bulb volume and olfactory sensitivity in patients with acute major depression. Neuroscience 169:415–421. [DOI] [PubMed] [Google Scholar]
- Negoias S, Hummel T, Symmank A, Schellong J, Joraschky P, Croy I(2016)Olfactory bulb volume predicts therapeutic outcome in major depression disorder. Brain Imaging Behav 10:367–372. [DOI] [PubMed] [Google Scholar]
- Pentzek M, Grass-Kapanke B, Ihl R(2007)Odor identification in alzheimer’s disease and depression. Aging Clin Exp Res 19:255–258. [DOI] [PubMed] [Google Scholar]
- Poulin SP, Dautoff R, Morris JC, Barrett LF, Dickerson BC, Alzheimer’s Disease Neuroimaging Initiative (2011)Amygdala atrophy is prominent in early alzheimer’s disease and relates to symptom severity. Psychiatry Res 194:7–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rahayel S, Frasnelli J, Joubert S(2012)The effect of Alzheimer’s disease and Parkinson’s disease on olfaction: a meta-analysis. Behav Brain Res 231:60–74. [DOI] [PubMed] [Google Scholar]
- Roberts RO, Christianson TJ, Kremers WK, Mielke MM, Machulda MM, Vassilaki M, Alhurani RE, Geda YE, Knopman DS, Petersen RC(2016)Association between olfactory dysfunction and amnestic mild cognitive impairment and alzheimer disease dementia. JAMA Neurol 73:93–101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Solomon GS, Petrie WM, Hart JR, Brackin HB Jr(1998)Olfactory dysfunction discriminates alzheimer’s dementia from major depression. J Neuropsychiatry Clin Neurosci 10:64–67. [DOI] [PubMed] [Google Scholar]
- Thomann PA, Dos Santos V, Toro P, Schönknecht P, Essig M, Schröder J(2009)Reduced olfactory bulb and tract volume in early Alzheimer’s disease–a MRI study. Neurobiol Aging 30:838–841. [DOI] [PubMed] [Google Scholar]
- Tombaugh TN, McIntyre NJ(1992)The mini-mental state examination: a comprehensive review. J Am Geriatr Soc 40:922–935. [DOI] [PubMed] [Google Scholar]
- Tzourio-Mazoyer N, Landeau B, Papathanassiou D, Crivello F, Etard O, Delcroix N, Mazoyer B, Joliot M(2002)Automated anatomical labeling of activations in SPM using a macroscopic anatomical parcellation of the MNI MRI single-subject brain. Neuroimage 15:273–289. [DOI] [PubMed] [Google Scholar]
- Wang J, Eslinger PJ, Doty RL, Zimmerman EK, Grunfeld R, Sun X, Meadowcroft MD, Connor JR, Price JL, Smith MB, Yang QX(2010)Olfactory deficit detected by fmri in early Alzheimer’s disease. Brain Res 1357:184–194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang S, Blazer DG(2015)Depression and cognition in the elderly. Annu Rev Clin Psychol 11:331–360. [DOI] [PubMed] [Google Scholar]
- Wang S, Dan GB(2014)Depression and cognition in the elderly. Annu Rev Clin Psychol 11:331–60. [DOI] [PubMed] [Google Scholar]
- Weyerer S, Eifflaender-Gorfer S, Köhler L, Jessen F, Maier W, Fuchs A, Pentzek M, Kaduszkiewicz H, Bachmann C, Angermeyer MC(2005)Prevalence and risk factors for depression in non-demented primary care attenders aged 75 years and older. J Affect Disord 111:153–163. [DOI] [PubMed] [Google Scholar]
- Woods SW.(2003)Chlorpromazine equivalent doses for the newer atypical antipsychotics. J Clin Psychiatry 64:663–667. [DOI] [PubMed] [Google Scholar]
- Yang J, Pan P, Song W, Huang R, Li J, Chen K, Gong Q, Zhong J, Shi H, Shang H(2012)Voxelwise meta-analysis of gray matter anomalies in alzheimer’s disease and mild cognitive impairment using anatomic likelihood estimation. J Neurol Sci 316:21–29. [DOI] [PubMed] [Google Scholar]
- Yousem DM, Geckle RJ, Bilker WB, Doty RL(1998)Olfactory bulb and tract and temporal lobe volumes. Normative data across decades. Ann N Y Acad Sci 855:546–555. [DOI] [PubMed] [Google Scholar]
- Yu H, Purgato M, Magni LR, Ogawa Y, Takeshima N, Cipriani A, Barbui C, Leucht S, Furukawa TA(2015)Dose equivalents of antidepressants: evidence-based recommendations from randomized controlled trials. J Affect Disord 180:179. [DOI] [PubMed] [Google Scholar]
- Zhang C, Wang X(2017)Initiation of the age-related decline of odor identification in humans: a meta-analysis. Ageing Res Rev 40:45–50. [DOI] [PubMed] [Google Scholar]